1,2,3,4,7-Pentamethoxy-9H-xanthen-9-oneCAS# 14254-96-7 |
Quality Control & MSDS
Number of papers citing our products

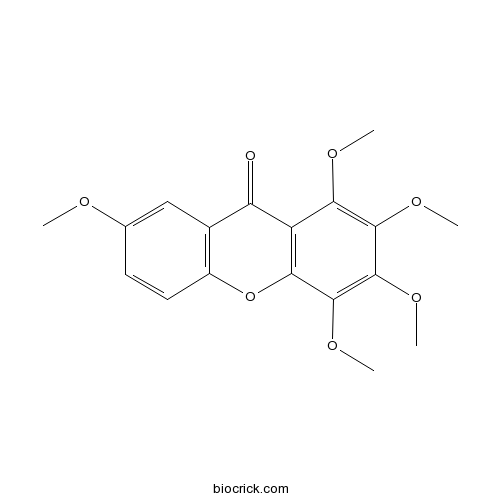
Chemical structure

3D structure
| Cas No. | 14254-96-7 | SDF | Download SDF |
| PubChem ID | 14528824 | Appearance | Yellow powder |
| Formula | C18H18O7 | M.Wt | 346.33 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,2,3,4,7-pentamethoxyxanthen-9-one | ||
| SMILES | COC1=CC2=C(C=C1)OC3=C(C2=O)C(=C(C(=C3OC)OC)OC)OC | ||
| Standard InChIKey | DHPUCRJALNWKKQ-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

1,2,3,4,7-Pentamethoxy-9H-xanthen-9-one Dilution Calculator

1,2,3,4,7-Pentamethoxy-9H-xanthen-9-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8874 mL | 14.4371 mL | 28.8742 mL | 57.7484 mL | 72.1855 mL |
| 5 mM | 0.5775 mL | 2.8874 mL | 5.7748 mL | 11.5497 mL | 14.4371 mL |
| 10 mM | 0.2887 mL | 1.4437 mL | 2.8874 mL | 5.7748 mL | 7.2185 mL |
| 50 mM | 0.0577 mL | 0.2887 mL | 0.5775 mL | 1.155 mL | 1.4437 mL |
| 100 mM | 0.0289 mL | 0.1444 mL | 0.2887 mL | 0.5775 mL | 0.7219 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-690,330
Catalog No.:BCC5666
CAS No.:142523-38-4
- L-690,488
Catalog No.:BCC5667
CAS No.:142523-14-6
- MK-5172 sodium salt
Catalog No.:BCC1765
CAS No.:1425038-27-2
- 19-Nortestosterone acetate
Catalog No.:BCC8445
CAS No.:1425-10-1
- Glyasperin A
Catalog No.:BCN6228
CAS No.:142474-52-0
- 3-O-beta-D-apiofuranosyl(1-2)-beta-D-glucopyranosyl rhamnocitrin 4-O-beta-D-glucopyranoside
Catalog No.:BCN8141
CAS No.:142473-99-2
- Amthamine dihydrobromide
Catalog No.:BCC6744
CAS No.:142457-00-9
- Lobetyolinin
Catalog No.:BCN3322
CAS No.:142451-48-7
- Myricetin 3-O-beta-D-xylopyranosyl(1-2)-beta-D-glucopyranoside
Catalog No.:BCN8140
CAS No.:142449-93-2
- NHS-SS-Biotin
Catalog No.:BCC3581
CAS No.:142439-92-7
- Crovatin
Catalog No.:BCN2517
CAS No.:142409-09-4
- Mesterolone
Catalog No.:BCC9023
CAS No.:1424-00-6
- Cimidahurinine
Catalog No.:BCN6229
CAS No.:142542-89-0
- Sageone
Catalog No.:BCN3144
CAS No.:142546-15-4
- A 484954
Catalog No.:BCC6203
CAS No.:142557-61-7
- Glyasperin D
Catalog No.:BCN6836
CAS No.:142561-10-2
- Calanolide E
Catalog No.:BCN6230
CAS No.:142566-61-8
- Asperuloside
Catalog No.:BCN6231
CAS No.:14259-45-1
- Narirutin
Catalog No.:BCN6300
CAS No.:14259-46-2
- Didymin
Catalog No.:BCN3327
CAS No.:14259-47-3
- Deacetylasperulosidic acid
Catalog No.:BCN3323
CAS No.:14259-55-3
- Daphylloside
Catalog No.:BCN6232
CAS No.:14260-99-2
- Macrocarpal C
Catalog No.:BCN6233
CAS No.:142628-53-3
- Macrocarpal E
Catalog No.:BCN6234
CAS No.:142628-54-4
ZnBr2-Mediated oxidative spiro-bromocyclization of propiolamide for the synthesis of 3-bromo-1-azaspiro[4.5]deca-3,6,9-triene-2,8-dione.[Pubmed:28379277]
Org Biomol Chem. 2017 Apr 18;15(16):3485-3490.
ZnBr2-Mediated oxidative spiro-bromocyclization of N-arylpropiolamide has been described herein for the synthesis of 3-bromo-1-azaspiro[4.5]deca-3,6,9-triene-2,8-dione with high efficiency. One equivalent of water was introduced into the final product. The reaction efficiently proceeded at room temperature, and an excellent tolerance of functional groups was demonstrated. Under standard conditions, 3-bromo-1-oxaspiro[4.5]deca-3,6,9-triene-2,8-dione and 3-bromo-1-azaspiro[4.5]deca-3,6,9-trien-8-one were synthesized.
Structural and Functional State of Erythrocyte Membranes in Mice at Different Stages of Experimental Parkinson's Disease Induced by Administration of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP).[Pubmed:28382410]
Bull Exp Biol Med. 2017 Mar;162(5):597-601.
We studied some structural and functional parameters of erythrocyte membranes in mice at the late presymptomatic and early symptomatic stages of experimental Parkinson's disease induced by administration of MPTP (hemolysis, microviscosity of different regions of the lipid bilayer, LPO intensity, activity of antioxidant enzymes, and kinetic properties of acetylcholinesterase). At the presymptomatic stage, significant deviations of the studied parameters from the normal were observed; they were similar in direction and magnitude to those in humans with Parkinson's disease. At the early symptomatic stage, most parameters tended to normal. Microviscosity of bulk lipids increased at the presymptomatic stage and decreased after appearance of clinical symptoms. This dynamics probably reflects activation of compensatory mechanisms aimed at inhibition of oxidative stress triggered by the development of the pathological process.
Synthesis, Characterization and Cytotoxicity of Substituted [1]Benzothieno[3,2-e][1,2,4]triazolo [4,3-a]pyrimidines.[Pubmed:28380219]
Acta Chim Slov. 2017 Mac;64(1):102-116.
A new series of 4-benzyl-6,7,8,9-tetrahydro[1]benzothieno[3,2-e][1,2,4]triazolo[4,3-a]pyrimidines was synthesized motivated by the widely reported anticancer activity of thieno[2,3-d]pyrimidines and triazolothienopyrimidines. The in vitro cytotoxic activity of some selected compounds was evaluated against two human cell lines: prostate cancer (PC-3) and colon cancer (HCT-116). A preliminary study of the structure-activity relationship of the target compounds was discussed. Most of the synthesized compounds showed remarkable activity on the tested cell lines, while compound 16c had the highest potency against the PC-3 cell line with an IC50 of 5.48 muM compared to Doxorubicin (IC50 = 7.7 muM), the reference standard used in this study. On the other hand, 6c and 18c were the most active against HCT-116 (IC50 = 6.12 and 6.56 muM, respectively) relative to IC50 = 15.82 muM of the standard. Thus, some of the synthesized thienopyrimidine derivatives, specially 6c, 16c and 18c, have the potential to be developed into potent anticancer agents.
A formal intermolecular [4 + 2] cycloaddition reaction of 1,3-disubstituted indoles and alkylquinones.[Pubmed:28379272]
Org Biomol Chem. 2017 Apr 18;15(16):3472-3478.
A formal [4 + 2] cycloaddition reaction of 1,3-disubstituted indoles and alkylquinones was realized to furnish polycyclic indolines in good yields. This protocol proceeded smoothly under basic conditions, with high atom-economy and broad substrate scope.


