10-Hydroxycanthin-6-oneCAS# 86293-41-6 |
Quality Control & MSDS
Number of papers citing our products

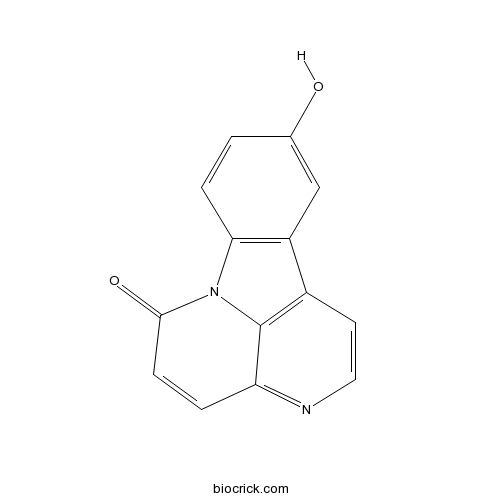
Chemical structure

3D structure
| Cas No. | 86293-41-6 | SDF | Download SDF |
| PubChem ID | 158929 | Appearance | Yellow powder |
| Formula | C14H8N2O2 | M.Wt | 236.23 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | C1=CC2=C(C=C1O)C3=C4N2C(=O)C=CC4=NC=C3 | ||
| Standard InChIKey | FZLISVGXWLVEPB-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 10-Hydroxycanthin-6-one has potential to be an antimicrobial agent. 2. 10-Hydroxycanthin-6-one has in vitro antimalarial activity. 3. 10-Hydroxycanthin-6-one has cytotoxic activity, it could be a plant anticancer agent. |
| Targets | Antifection |

10-Hydroxycanthin-6-one Dilution Calculator

10-Hydroxycanthin-6-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2332 mL | 21.1658 mL | 42.3316 mL | 84.6633 mL | 105.8291 mL |
| 5 mM | 0.8466 mL | 4.2332 mL | 8.4663 mL | 16.9327 mL | 21.1658 mL |
| 10 mM | 0.4233 mL | 2.1166 mL | 4.2332 mL | 8.4663 mL | 10.5829 mL |
| 50 mM | 0.0847 mL | 0.4233 mL | 0.8466 mL | 1.6933 mL | 2.1166 mL |
| 100 mM | 0.0423 mL | 0.2117 mL | 0.4233 mL | 0.8466 mL | 1.0583 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isoiridogermanal
Catalog No.:BCN7613
CAS No.:86293-25-6
- Ganoderic acid TR
Catalog No.:BCN3207
CAS No.:862893-75-2
- Salvianan
Catalog No.:BCN3545
CAS No.:862832-46-0
- IKK-3 Inhibitor
Catalog No.:BCC1643
CAS No.:862812-98-4
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
- Imatinib hydrochloride
Catalog No.:BCC1644
CAS No.:862366-25-4
- RA VII
Catalog No.:BCN3512
CAS No.:86229-97-2
- Valeriotriate B
Catalog No.:BCN6751
CAS No.:862255-64-9
- Mirodenafil
Catalog No.:BCC5254
CAS No.:862189-95-5
- Nandrolone undecylate
Catalog No.:BCC9090
CAS No.:862-89-5
- Anamorelin hydrochloride
Catalog No.:BCC1364
CAS No.:861998-00-7
- 7-Methoxy-3,4-dihydro-1-naphthalenylacetonitrile
Catalog No.:BCC8781
CAS No.:861960-34-1
- alpha-Amyrin acetate
Catalog No.:BCN4410
CAS No.:863-76-3
- Azilsartan Medoxomil
Catalog No.:BCC5021
CAS No.:863031-21-4
- Azilsartan medoxomil monopotassium
Catalog No.:BCC4089
CAS No.:863031-24-7
- Impurity C of Calcitriol
Catalog No.:BCC5384
CAS No.:86307-44-0
- Dasatinib monohydrate
Catalog No.:BCN2177
CAS No.:863127-77-9
- 8-epi-Chlorajapolide F
Catalog No.:BCN6426
CAS No.:863301-69-3
- PSI-6206
Catalog No.:BCC3609
CAS No.:863329-66-2
- Medetomidine
Catalog No.:BCC1736
CAS No.:86347-14-0
- Medetomidine HCl
Catalog No.:BCC4351
CAS No.:86347-15-1
- Cebranopadol
Catalog No.:BCC1467
CAS No.:863513-91-1
- Gnetol
Catalog No.:BCN3382
CAS No.:86361-55-9
- 6-Epiharpagide
Catalog No.:BCN4563
CAS No.:86362-16-5
Isolation, structural elucidation and cytotoxicity evaluation of a new pentahydroxy-pimarane diterpenoid along with other chemical constituents from Aerva lanata.[Pubmed:25348942]
Nat Prod Res. 2015 Feb;29(3):253-61.
Aervalanata possesses various useful medicinal and pharmaceutical activities. Phytochemical investigation of the plant has now led to the isolation of a new 2alpha,3alpha,15,16,19-pentahydroxy pimar-8(14)-ene diterpenoid (1) together with 12 other known compounds identified as beta-sitosterol (2), beta-sitosterol-3-O-beta-D-glucoside (3), canthin-6-one (4), 10-Hydroxycanthin-6-one (aervine, 5), 10-methoxycanthin-6-one (methylaervine, 6), beta-carboline-1-propionic acid (7), 1-O-beta-D-glucopyranosyl-(2S,3R,8E)-2-[(2'R)-2-hydroxylpalmitoylamino]-8-octadec ene-1,3-diol (8), 1-O-(beta-D-glucopyranosyl)-(2S,3S,4R,8Z)-2-[(2'R)-2'-hydroxytetracosanoylamino]- 8(Z)-octadene-1,3,4-triol (9), (2S,3S,4R,10E)-2-[(2'R)-2'-hydroxytetracosanoylamino]-10-octadecene-1,3,4-triol (10), 6'-O-(4''-hydroxy-trans-cinnamoyl)-kaempferol-3-O-beta-D-glucopyranoside (tribuloside, 11), 3-cinnamoyltribuloside (12) and sulfonoquinovosyldiacylglyceride (13). Among these, six compounds (8-13) are reported for the first time from this plant. Cytotoxicity evaluation of the compounds against five cancer cell lines (CHO, HepG2, HeLa, A-431 and MCF-7) shows promising IC50 values for compounds 4, 6 and 12.
Synthesis and Evaluation of Ester Derivatives of 10-Hydroxycanthin-6-one as Potential Antimicrobial Agents.[Pubmed:27007362]
Molecules. 2016 Mar 21;21(3):390.
As part of our continuing research on canthin-6-one antimicrobial agents, a new series of ester derivatives of 10-Hydroxycanthin-6-one were synthesized using a simple and effective synthetic route. The structure of each compound was characterized by NMR, ESI-MS, FT-IR, UV, and elemental analysis. The antimicrobial activity of these compounds against three phytopathogenic fungi (Alternaria solani, Fusarium graminearum, and Fusarium solani) and four bacteria (Bacillus cereus, Bacillus subtilis, Ralstonia solanacearum, and Pseudomonas syringae) were evaluated using the mycelium linear growth rate method and micro-broth dilution method, respectively. The structure-activity relationship is discussed. Of the tested compounds, 4 and 7s displayed significant antifungal activity against F. graminearum, with inhibition rates of 100% at a concentration of 50 mug/mL. Compounds 5, 7s, and 7t showed the best inhibitory activity against all the tested bacteria, with minimum inhibitory concentrations (MICs) between 3.91 and 31.25 mug/mL. Thus, 7s emerged as a promising lead compound for the development of novel canthine-6-one antimicrobial agents.


