17 alpha-propionateAndrogen antagonist CAS# 19608-29-8 |
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- Andarine
Catalog No.:BCC1168
CAS No.:401900-40-1
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure
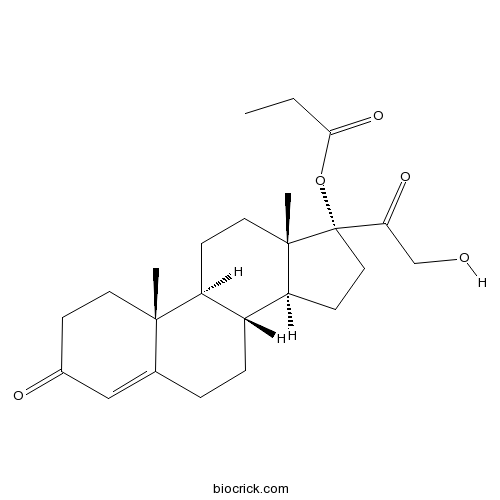
3D structure
| Cas No. | 19608-29-8 | SDF | Download SDF |
| PubChem ID | 11750009 | Appearance | Powder |
| Formula | C24H34O5 | M.Wt | 402.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (248.43 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(8R,9S,10R,13S,14S,17R)-17-(2-hydroxyacetyl)-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl] propanoate | ||
| SMILES | CCC(=O)OC1(CCC2C1(CCC3C2CCC4=CC(=O)CCC34C)C)C(=O)CO | ||
| Standard InChIKey | GPNHMOZDMYNCPO-PDUMRIMRSA-N | ||
| Standard InChI | InChI=1S/C24H34O5/c1-4-21(28)29-24(20(27)14-25)12-9-19-17-6-5-15-13-16(26)7-10-22(15,2)18(17)8-11-23(19,24)3/h13,17-19,25H,4-12,14H2,1-3H3/t17-,18+,19+,22+,23+,24+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

17 alpha-propionate Dilution Calculator

17 alpha-propionate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4843 mL | 12.4217 mL | 24.8435 mL | 49.687 mL | 62.1087 mL |
| 5 mM | 0.4969 mL | 2.4843 mL | 4.9687 mL | 9.9374 mL | 12.4217 mL |
| 10 mM | 0.2484 mL | 1.2422 mL | 2.4843 mL | 4.9687 mL | 6.2109 mL |
| 50 mM | 0.0497 mL | 0.2484 mL | 0.4969 mL | 0.9937 mL | 1.2422 mL |
| 100 mM | 0.0248 mL | 0.1242 mL | 0.2484 mL | 0.4969 mL | 0.6211 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Acne vulgaris is a disorder of the pilosebaceous unit in which the androgens contribute to its onset and persistence. Though the use of antiandrogens is potentially effective; topical use of antiandrogens are not available on the market. Cortexolone 17a-propionate (CB-03-01) is a new potent topical antiandrogen potentially useful in acne vulgaris. CB-03-01 is a new chemical entity that acts at the level of the skin androgen receptor, which blocks testosterone and di-hydrotestosterone from binding to the receptor in the cell.
In vitro: The aim of one in vitro study was to investigate the antiandrogenic activity of a new monoester of cortexolone, cortexolone 17alpha-propionate. Although the compound displayed a strong local antiandrogenic activity in hamster's flank organ test. Its pharmacological activity seemed to be primarily related to its ability to antagonistically compete at androgen receptor level; nevertheless its primary pharmacological target needs to be further investigated. The topical activity of cortexolone 17alpha-propionate with the apparent absence of systemic effects makes this compound to have the potential of representing a novel and safe therapeutic approach for androgen-dependent skin disorders. [1].
In vivo: Cortexolone 17alpha-propionate displayed a strong local antiandrogenic activity in hamster's flank organ test, however, it did not exhibit antiandrogenic activity in rats, nor did it affect gonadotropins hypersecretion. As topical antiandrogen, the steroid resulted about 4 times more active than progesterone and, when compared to known antiandrogen standards, it was about 3 times more potent than flutamide, about 2 times more effective than finasteride and approximately as active as cyproterone acetate. Its pharmacological activity seemed to be primarily related to its ability to antagonistically compete at androgen receptor level; nevertheless its primary pharmacological target needs to be further investigated [1].
Clinical trial: In 2011, Trifu et al. evaluated the safety and efficacy of cortexolone 17alpha-propionate 1% cream in acne vulgaris in comparison to placebo and to tretinoin 0.05% cream. A total of 77 male subjects were randomized to receive cortexolone 17alpha-propionate 1% cream, tretinoin 0.05% cream or placebo once nightly for 8 weeks. cortexolone 17alpha-propionate 1% cream was statistically better than placebo in reducing total lesion count, inflammatory lesion count and Acne Severity Index, without any major side effects. Further, cortexolone 17alpha-propionate 1% cream showed a faster onset of all the abovementioned improvements and was clinically more effective than tretinoin 0.05% cream, although the results were not statistically significant [2]
Reference:
[1] Celasco G, Moro L, Bozzella R, Ferraboschi P, Bartorelli L, Quattrocchi C, Nicoletti F. Biological profile of cortexolone 17alpha-propionate (CB-03-01), a new topical and peripherally selective androgen antagonist. Arzneimittelforschung. 2004;54(12):881-6.
[2] Trifu V, Tiplica GS, Naumescu E, Zalupca L, Moro L, Celasco G. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0•05% cream. Br J Dermatol. 2011;165(1):177-83.
- 25S-Inokosterone
Catalog No.:BCN3873
CAS No.:19595-18-7
- (R)-Nepicastat HCl
Catalog No.:BCC4315
CAS No.:195881-94-8
- HTMT dimaleate
Catalog No.:BCC6736
CAS No.:195867-54-0
- 2,6,16-Kauranetriol 2-O-beta-D-allopyranoside
Catalog No.:BCN1509
CAS No.:195735-16-1
- Atrasentan hydrochloride
Catalog No.:BCC1380
CAS No.:195733-43-8
- (+-)-Byakangelicin
Catalog No.:BCN5000
CAS No.:19573-01-4
- 2,16,19-Kauranetriol 2-O-beta-D-allopyranoside
Catalog No.:BCN1510
CAS No.:195723-38-7
- Methyl 4-O-feruloylquinate
Catalog No.:BCC9041
CAS No.:195723-10-5
- Piromidic Acid
Catalog No.:BCC3840
CAS No.:19562-30-2
- AP1903
Catalog No.:BCC5361
CAS No.:195514-63-7
- Chiirirhamnin
Catalog No.:BCN3179
CAS No.:195450-50-1
- Wortmannin
Catalog No.:BCC3874
CAS No.:19545-26-7
- Angelol A
Catalog No.:BCN8036
CAS No.:19625-17-3
- Indole-3-acrylic acid methyl ester
Catalog No.:BCN1190
CAS No.:19626-92-7
- 4-Methoxycinnamaldehyde
Catalog No.:BCN2700
CAS No.:1963-36-6
- Bay 11-7085
Catalog No.:BCC5105
CAS No.:196309-76-9
- Axillarine
Catalog No.:BCN2059
CAS No.:19637-66-2
- Prosaptide TX14(A)
Catalog No.:BCC8020
CAS No.:196391-82-9
- Siegesmethyethericacid
Catalog No.:BCC9248
CAS No.:196399-16-3
- (4S,5R)-3-tert-butoxycarbony-2-(4-anisy)-4-phenyl-5-oxazolidinecarboxylic acid
Catalog No.:BCN8365
CAS No.:196404-55-4
- (RS)-3,5-DHPG
Catalog No.:BCC6613
CAS No.:19641-83-9
- 2S-Amino-3R-octadecanol
Catalog No.:BCN1775
CAS No.:196497-48-0
- 4-Acetyl Ramelteon
Catalog No.:BCC1107
CAS No.:1346598-94-4
- BIBX 1382
Catalog No.:BCC1418
CAS No.:196612-93-8
Biotransformation of the topical glucocorticoids budesonide and beclomethasone 17 alpha,21-dipropionate in human liver and lung homogenate.[Pubmed:6150981]
J Pharm Pharmacol. 1984 Nov;36(11):763-5.
Tritiated budesonide and beclomethasone 17 alpha,21-dipropionate (BDP) were incubated with the 9000g supernatant of human liver homogenate. BDP was immediately hydrolysed to beclomethasone 17 alpha-propionate (BMP). BMP was then further biotransformed to polar metabolites. Budesonide was rapidly biotransformed (2-4 times more rapidly than BMP) to metabolites of low glucocorticoid potency. The compounds were also incubated with the 1000g supernatant of human lung homogenate. BDP was rapidly hydrolysed to BMP and then more slowly to beclomethasone. Budesonide was not biotransformed in the lung.
Glucocorticoid resistant asthma: T-lymphocyte steroid metabolism and sensitivity to glucocorticoids and immunosuppressive agents.[Pubmed:8902470]
Eur Respir J. 1996 Oct;9(10):2077-86.
We have previously shown that T-lymphocytes from clinically glucocorticoid (GC) resistant asthmatics are more refractory to dexamethasone suppression in vitro than those of GC sensitive asthmatics. We wished to extend these observations to compare three GCs used topically for asthma therapy (budesonide, beclomethasone dipropionate and fluticasone 17 alpha-propionate) and three immunosuppressive drugs (cyclosporin A, FK506 (tacrolimus) and mycophenolate mofetil) with dexamethasone for their antiproliferative effects on T-lymphocytes from GC sensitive and resistant asthmatics, and also to compare the rates of steroid metabolism by T-lymphocytes from these patients. Antiproliferative activity of the drugs was measured on peripheral blood T-lymphocytes activated with phytohaemagglutinin (PHA) and anti-CD3 antibody in vitro. The rates of total steroid metabolism and 20 alpha-hydroxylation by T-cell homogenates were measured using radiolabelled progesterone as an established probe substrate. Over a wide concentration range, T-lymphocytes from GC resistant asthmatics were significantly less inhibited by all four GCs as compared with cells from GC sensitive asthmatics. The median inhibitory concentrations (IC50) for inhibition of T-lymphocytes from the GC resistant asthmatics exceeded those likely to be achieved therapeutically by systemic administration (although higher concentrations might in theory be achieved locally in the bronchial mucosa by inhaled administration). In contrast, all three immunosuppressive drugs at putative therapeutic concentrations inhibited T-lymphocytes both from GC sensitive and resistant asthmatics with equivalent potency. The rates of total metabolism and 20 alpha-hydroxylation of steroid by homogenates of T-lymphocytes from GC sensitive and resistant asthmatics were equivalent. Thus, relative GC resistance in T-lymphocytes from GC resistant as compared with sensitive asthmatics is: 1) manifest with GC molecules of variable molecular structure; 2) not accompanied by elevated intracellular metabolism of steroids; and 3) overcome by immunosuppressive drugs which inhibit T-lymphocytes by non-GC-mediated mechanisms. We conclude that current anti-asthma glucocorticoids at therapeutic concentrations are unlikely to be of benefit for the therapy of glucocorticoid resistant asthma, and that other immunosuppressive drugs may have potential as therapeutic agents in these patients.
Emerging drugs for the treatment of acne.[Pubmed:25474485]
Expert Opin Emerg Drugs. 2015 Mar;20(1):91-101.
INTRODUCTION: Acne is the most common skin condition in the US. The mainstay of acne therapy includes: topical retinoids, topical antibiotics, benzoyl peroxide (BP), and oral isotretinoin for severe cases. Although these treatment options are highly effective they do have certain drawbacks. Current acne treatment regimens often require patients to use multiple medications, some of which may have irritating side effects. Furthermore, Propionibacterium acnes resistance to antibiotics has become an increasing problem due to the rise in antibiotic use. AREAS COVERED: New therapies that have either been released onto the market or that are being developed include: adapalene-BP combination agent, dapsone 5% gel, minocycline foam, topical nitric oxide-releasing agent, cortexolone 17 alpha-propionate, and CIP isotretinoin. Some of these new therapies address the challenges faced with existing treatment options. For instance, the relatively new combination therapy, adapalene-BP, limits antibiotic resistance and also helps simplify treatment regimens. The newly developed topical nitric oxide-releasing agent also holds potential in limiting antibiotic resistance. EXPERT OPINION: Many of the new therapies discussed in this paper are still in early stages of testing so it is difficult to predict their outlook; however, based on preliminary findings, these therapies seem to be promising.


