2,6-Dimethoxybenzoic acidCAS# 1466-76-8 |
Quality Control & MSDS
Number of papers citing our products

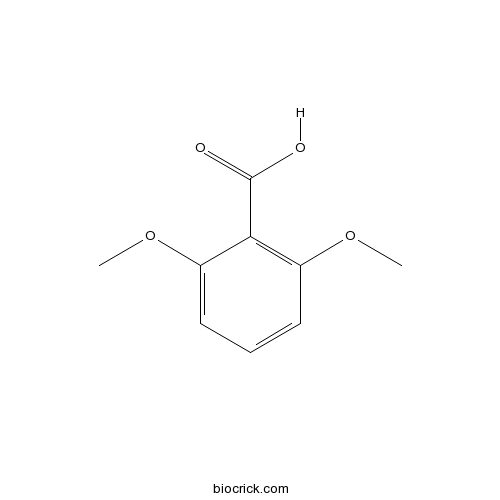
Chemical structure

3D structure
| Cas No. | 1466-76-8 | SDF | Download SDF |
| PubChem ID | 15109 | Appearance | Powder |
| Formula | C9H10O4 | M.Wt | 182.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,6-dimethoxybenzoic acid | ||
| SMILES | COC1=C(C(=CC=C1)OC)C(=O)O | ||
| Standard InChIKey | MBIZFBDREVRUHY-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Benzyl 2,6-dimethoxybenzoate shows antimicrobial activity against several bacteria (S. aureus, E. coli, P. aeruginosa, and B. subtilis) and fungi (C. albicans and T. mentagrophytes). |
| Targets | Antifection |
| In vitro | New furanoid diterpenes from Caesalpinia pulcherrima.[Pubmed: 12193012]J Nat Prod. 2002 Aug;65(8):1107-10.Four new cassane-type furanoditerpenoids (1-4) were isolated from the air-dried leaves of Caesalpinia pulcherrima. Their structures were elucidated by spectral data interpretation. The exocyclic methylene compound 1 readily isomerized and oxidized to the benzofuran 4. Benzyl 2,6-dimethoxybenzoate (5) was also identified in this study. Antimicrobial tests on 1-5 indicated that they were active against several bacteria (S. aureus, E. coli, P. aeruginosa, and B. subtilis) and fungi (C. albicans and T. mentagrophytes). |
| Structure Identification | Acta Crystallogr C. 2012 Nov;68(Pt 11):o447-51.Supramolecular association in proton-transfer adducts containing benzamidinium cations. II. Concomitant polymorphs of the molecular salt of 2,6-dimethoxybenzoic acid with benzamidine.[Pubmed: 23124460]Two concomitant polymorphs of the molecular salt formed by 2,6-Dimethoxybenzoic acid, C(9)H(10)O(4) (Dmb), with benzamidine, C(7)H(8)N(2) (benzenecarboximidamide, Benzam) from water solution have been identified. Benzamidinidium 2,6-dimethoxybenzoate, C(7)H(9)N(2)(+)·C(9)H(9)O(4)(-) (BenzamH(+)·Dmb(-)), was obtained through protonation at the imino N atom of Benzam as a result of proton transfer from the acidic hydroxy group of Dmb. In the monoclinic polymorph, (I) (space group P2(1)/n), the asymmetric unit consists of two Dmb(-) anions and two monoprotonated BenzamH(+) cations. In the orthorhombic polymorph, (II) (space group P2(1)2(1)2(1)), one Dmb(-) anion and one BenzamH(+) cation constitute the asymmetric unit. In both polymorphic salts, the amidinium fragments and carboxylate groups are completely delocalized. This delocalization favours the aggregation of the molecular components of these acid-base complexes into nonplanar dimers with an R(2)(2)(8) graph-set motif via N(+)-H···O(-) charge-assisted hydrogen bonding. Both the monoclinic and orthorhombic forms exhibit one-dimensional isostructurality, as the crystal structures feature identical hydrogen-bonding motifs consisting of dimers and catemers. J Pharm Biomed Anal. 2015 Jan;102:236-45.Qualitative and quantitative analysis on chemical constituents from Curculigo orchioides using ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry.[Pubmed: 25305598]A rapid ultra-high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UHPLC-ESI-Q-TOF/MS) method was developed for qualitative and quantitative determination of constituents in the rhizome of Curculigo orchioides.
|

2,6-Dimethoxybenzoic acid Dilution Calculator

2,6-Dimethoxybenzoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4885 mL | 27.4424 mL | 54.8847 mL | 109.7695 mL | 137.2119 mL |
| 5 mM | 1.0977 mL | 5.4885 mL | 10.9769 mL | 21.9539 mL | 27.4424 mL |
| 10 mM | 0.5488 mL | 2.7442 mL | 5.4885 mL | 10.9769 mL | 13.7212 mL |
| 50 mM | 0.1098 mL | 0.5488 mL | 1.0977 mL | 2.1954 mL | 2.7442 mL |
| 100 mM | 0.0549 mL | 0.2744 mL | 0.5488 mL | 1.0977 mL | 1.3721 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Cyclopropyl-3-[(diphenylphosphinyl)methyl]-4-(4-fluorophenyl)quinoline
Catalog No.:BCC8572
CAS No.:146578-99-6
- Fmoc-Gly(allyl)-OH
Catalog No.:BCC3156
CAS No.:146549-21-5
- Tyrphostin AG 1296
Catalog No.:BCC1195
CAS No.:146535-11-7
- WR 1065
Catalog No.:BCC2417
CAS No.:14653-77-1
- Complanatoside A
Catalog No.:BCN6282
CAS No.:146501-37-3
- 1-Methylpsilocin
Catalog No.:BCC7536
CAS No.:1465-16-3
- Pralatrexate
Catalog No.:BCC2304
CAS No.:146464-95-1
- Camaric acid
Catalog No.:BCN1650
CAS No.:146450-83-1
- Flavopiridol
Catalog No.:BCC1577
CAS No.:146426-40-6
- Lactose
Catalog No.:BCN8387
CAS No.:14641-93-1
- Desmethylrocaglamide
Catalog No.:BCN7735
CAS No.:146408-78-8
- SR 48692
Catalog No.:BCC7763
CAS No.:146362-70-1
- Dantrolene, sodium salt
Catalog No.:BCC6673
CAS No.:14663-23-1
- H-Trp(Boc)-OH
Catalog No.:BCC3115
CAS No.:146645-63-8
- SR 2640 hydrochloride
Catalog No.:BCC7180
CAS No.:146662-42-2
- (RS)-MCPG
Catalog No.:BCC6610
CAS No.:146669-29-6
- SR 11237
Catalog No.:BCC7681
CAS No.:146670-40-8
- Hybridaphniphylline A
Catalog No.:BCN7042
CAS No.:1467083-07-3
- Hybridaphniphylline B
Catalog No.:BCN7045
CAS No.:1467083-09-5
- Daphnicyclidin I
Catalog No.:BCN7038
CAS No.:1467083-10-8
- ZD 7155 hydrochloride
Catalog No.:BCC5734
CAS No.:146709-78-6
- Boc-D-Phe(4-CN)-OH
Catalog No.:BCC3183
CAS No.:146727-62-0
- N-desmethyldauricine
Catalog No.:BCC8217
CAS No.:146763-55-5
- Y-29794 oxalate
Catalog No.:BCC5795
CAS No.:146794-84-5
Supramolecular association in proton-transfer adducts containing benzamidinium cations. II. Concomitant polymorphs of the molecular salt of 2,6-dimethoxybenzoic acid with benzamidine.[Pubmed:23124460]
Acta Crystallogr C. 2012 Nov;68(Pt 11):o447-51.
Two concomitant polymorphs of the molecular salt formed by 2,6-Dimethoxybenzoic acid, C(9)H(10)O(4) (Dmb), with benzamidine, C(7)H(8)N(2) (benzenecarboximidamide, Benzam) from water solution have been identified. Benzamidinidium 2,6-dimethoxybenzoate, C(7)H(9)N(2)(+).C(9)H(9)O(4)(-) (BenzamH(+).Dmb(-)), was obtained through protonation at the imino N atom of Benzam as a result of proton transfer from the acidic hydroxy group of Dmb. In the monoclinic polymorph, (I) (space group P2(1)/n), the asymmetric unit consists of two Dmb(-) anions and two monoprotonated BenzamH(+) cations. In the orthorhombic polymorph, (II) (space group P2(1)2(1)2(1)), one Dmb(-) anion and one BenzamH(+) cation constitute the asymmetric unit. In both polymorphic salts, the amidinium fragments and carboxylate groups are completely delocalized. This delocalization favours the aggregation of the molecular components of these acid-base complexes into nonplanar dimers with an R(2)(2)(8) graph-set motif via N(+)-H...O(-) charge-assisted hydrogen bonding. Both the monoclinic and orthorhombic forms exhibit one-dimensional isostructurality, as the crystal structures feature identical hydrogen-bonding motifs consisting of dimers and catemers.
Fingerprint analysis and simultaneous determination of phenolic compounds in extracts of Curculiginis Rhizoma by HPLC-diode array detector.[Pubmed:23902862]
Chem Pharm Bull (Tokyo). 2013;61(8):802-8.
Curculiginis Rhizoma (Curculigo orchioides GAERTN.) is a well-known Chinese herbal medicine, as well as an important Rasayana drug in India. Current criteria of quality control on this herb are to quantitatively analyze single compound curculigoside, which fail to comprehensively evaluate quality of this herb. In this paper, a simple and reliable HPLC coupled with diode array detector (DAD) method was developed to evaluate the quality of Curculiginis Rhizoma through establishing chromatographic fingerprint and simultaneously quantitating four phenolic compounds, orcinol glucoside, orcinol, 2,6-Dimethoxybenzoic acid and curculigoside. The fingerprint displayed eleven common peaks, and the similarity index of different samples was in a range of 0.890-0.977. Validation of the method was acceptable, with 96.03-102.82% accuracy in recovery test and inter and intra-day precisions were less than 2%. This developed method by having a combination of chromatographic fingerprint and quantitation analysis could be applied to the quality control of Curculiginis Rhizoma.
Qualitative and quantitative analysis on chemical constituents from Curculigo orchioides using ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry.[Pubmed:25305598]
J Pharm Biomed Anal. 2015 Jan;102:236-45.
A rapid ultra-high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UHPLC-ESI-Q-TOF/MS) method was developed for qualitative and quantitative determination of constituents in the rhizome of Curculigo orchioides. Qualitative analysis was performed on a Waters ACQUITY UHPLC @ HSS T3 column (1.8 mum 100 x 2.1mm) using gradient elution with mobile phase of 0.1% formic acid and acetonitrile. Quantitative analysis was performed on an Agilent ZORBAX Eclipse plus C18 column (1.7 mum 100 x 2.1mm) using gradient elution with mobile phase of 0.1% acetic acid and acetonitrile for at least 20 min. Quadrupole TOF/MS in either full scan mode or extracted ion mode was used for qualitative and quantitative analysis of the constituents. According to the mass spectrometric fragmentation mechanism and UHPLC-ESI-Q-TOF-MS data, chemical structures of 45 constituents in the rhizome of Curculigo orchioides, including 19 phenols and phenolic glycosides, 16 lignans and lignan glycosides, 8 triterpenoid saponins, one flavone and one sesquiterpene, were identified tentatively on-line without the time-consuming process of isolation. In addition, 8 phenolic glycosides including 5-hydroxymethylfurfural (HMF), 2-hydroxy-5-(2-hydroxyethyl) phenyl-beta-D-glucopyranoside (HPG), anacardoside (ACD), orcinol glucoside (OGD), orcinol-1-O-beta-D-apiofuranosyl-(1 --> 6)-beta-D-glucopyranoside (OAG), 2,6-Dimethoxybenzoic acid (DBA), curculigoside (CUR) and curculigine A (CCL) were quantitated in 11 collected samples and 10 commercial samples from different providers. The results show that UHPLC-ESI-Q-TOF-MS is a viable method for analysis and quality evaluation of the constituents from the rhizome of Curculigo orchioides.
New furanoid diterpenes from Caesalpinia pulcherrima.[Pubmed:12193012]
J Nat Prod. 2002 Aug;65(8):1107-10.
Four new cassane-type furanoditerpenoids (1-4) were isolated from the air-dried leaves of Caesalpinia pulcherrima. Their structures were elucidated by spectral data interpretation. The exocyclic methylene compound 1 readily isomerized and oxidized to the benzofuran 4. Benzyl 2,6-dimethoxybenzoate (5) was also identified in this study. Antimicrobial tests on 1-5 indicated that they were active against several bacteria (S. aureus, E. coli, P. aeruginosa, and B. subtilis) and fungi (C. albicans and T. mentagrophytes).


