20(R)-ProtopanaxatriolCAS# 1453-93-6 |
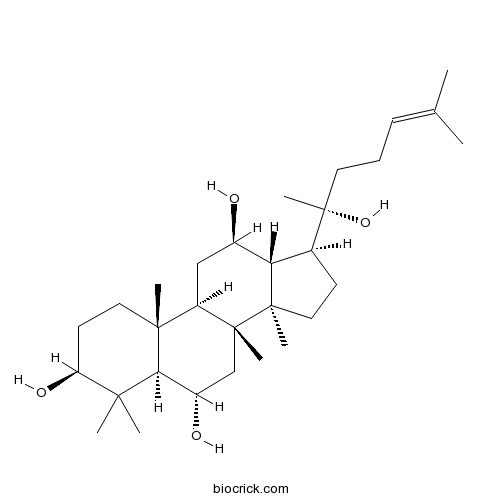
- Protopanaxatriol
Catalog No.:BCC9245
CAS No.:32773-56-1
- (20S)-Protopanaxatriol
Catalog No.:BCN2705
CAS No.:34080-08-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1453-93-6 | SDF | Download SDF |
| PubChem ID | 9847853 | Appearance | White powder |
| Formula | C30H52O4 | M.Wt | 476.73 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 20(R)-APPT; 20(R)-Protopanaxatriol | ||
| Solubility | DMSO : ≥ 32 mg/mL (67.12 mM); | ||
| Chemical Name | (3S,5R,6S,8R,9R,10R,12R,13R,14R,17S)-17-[(2R)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene-3,6,12-triol | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CC(C4C3(CCC(C4(C)C)O)C)O)C)O)C)O)C | ||
| Standard InChIKey | SHCBCKBYTHZQGZ-DLHMIPLTSA-N | ||
| Standard InChI | InChI=1S/C30H52O4/c1-18(2)10-9-13-30(8,34)19-11-15-28(6)24(19)20(31)16-22-27(5)14-12-23(33)26(3,4)25(27)21(32)17-29(22,28)7/h10,19-25,31-34H,9,11-17H2,1-8H3/t19-,20+,21-,22+,23-,24-,25-,27+,28+,29+,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 20(R)-Protopanaxatriol shows anti-hyperglycaemic, and anti-cancer activities.Protopanaxatriol has inhibitory effects on the enzyme catalytic activities of cyclooxygenases-1 and -2 (COX-1 and -2). |
| Targets | COX | PPAR |
| In vitro | Cyclooxygenase inhibitory activity of ginsenosides from heat-processed ginseng.[Reference: WebLink]Food Chem., 2012, 133(3):998-1000.Ginsenosides, from heat-processed ginseng, and sapogenins were evaluated for their inhibitory effects on the enzyme catalytic activities of cyclooxygenases-1 and -2 (COX-1 and -2). Semisynthesis and cytotoxicity evaluation of a series of ocotillol type saponins and aglycones from 20(S)-ginsenoside Rg2, Rh1, protopanaxatriol and their 20(R)-epimers[Reference: WebLink]Chemical Research in Chinese Universities, 2016, 32(1): 1-6.With the oxidation treatment, eighteen compounds were separated from 20(S)-ginsenoside Rg2, Rh1, protopanaxatriol(PPT) and their 20(R)-epimers in total and cytotoxicity of most of them was evaluated against three human cancer cell lines HeLa, A549 and MCF-7 by 3-(4,5-dimetylthiazol-z-yl)-2,5-diphenyltetrazolium bromide(MTT) assay. |
| Structure Identification | J. Funct. Foods, 2016, 23:188-97.The inhibition of α-glycosidase and protein tyrosine phosphatase 1B (PTP1B) activities by ginsenosides from Panax ginseng C.A. Meyer and simultaneous determination by HPLC-ELSD.[Reference: WebLink]Panax ginseng C.A. Meyer is extensively used as a food additive because of its medicinal and nutritional properties. |

20(R)-Protopanaxatriol Dilution Calculator

20(R)-Protopanaxatriol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0976 mL | 10.4881 mL | 20.9762 mL | 41.9525 mL | 52.4406 mL |
| 5 mM | 0.4195 mL | 2.0976 mL | 4.1952 mL | 8.3905 mL | 10.4881 mL |
| 10 mM | 0.2098 mL | 1.0488 mL | 2.0976 mL | 4.1952 mL | 5.2441 mL |
| 50 mM | 0.042 mL | 0.2098 mL | 0.4195 mL | 0.839 mL | 1.0488 mL |
| 100 mM | 0.021 mL | 0.1049 mL | 0.2098 mL | 0.4195 mL | 0.5244 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Protopanaxatriol is a natural PPARγ antagonist with an IC50 of 11.75 μM (Ki=4.2 μM).
In Vitro:Protopanaxatriol (PPT) does not show an inhibitory effect on other nuclear receptors involved in metabolic disorders, such as PPARα and PPARβ/σ, LXRα and LXRβ. A competitive binding assay using TR-FRET is performed. Protopanaxatriol is able to displace the Rosiglitazone from binding to PPARγ at an IC50 value of 11.75 μM (Ki=4.2 μM). Compared with unlabeled Rosiglitazone (Ki=43 nM), the PPARγ binding affinity of PPT is moderate[1]. To evaluate whether PPD, Protopanaxatriol (PPT), G-Rg3 and G-Rh2 regulate viability of lung cancer cells, the CCK-8 assay is performed to analysis the effect of these four compounds on the viability of A549 or SK-MES-1 cells. For A549 cells, all these compounds (PPD, Protopanaxatriol, G-Rg3 and G-Rh2) can notably inhibit the viability in a dosage-dependent manner (P<0.05, P<0.01 or P<0.001), especially at the concentration of 40 uM[2].
In Vivo:Protopanaxatriol, a novel PPARγ antagonist from Panax ginseng, alleviates steatosis in mice. onsistent with the increase of body temperature, the expression of uncoupling protein (UCP)-1, UCP-2 and UCP-3 genes, involved in energy dissipation and adoptive thermogenesis in brown adipose tissue (BAT), is enhanced in the brown adipose tissue (BAT) of PPT-treated mice. The other adipose tissue involving in the heat production and energy expenditure is beige adipose in WAT. The elevated expression of UCP-1 is a key feature for browning of WAT. Protopanaxatriol treatment increases the expression of UCP-1 in WAT[1].
References:
[1]. Zhang Y, et al. Protopanaxatriol, a novel PPARγ antagonist from Panax ginseng, alleviates steatosis in mice. Sci Rep. 2014 Dec 9;4:7375.
[2]. Xu FY, et al. The antitumor activity study of ginsenosides and metabolites in lung cancer cell. Am J Transl Res. 2016 Apr 15;8(4):1708-18.
- Diarylcomosol III
Catalog No.:BCN7201
CAS No.:1452487-93-2
- Sahandol
Catalog No.:BCN6996
CAS No.:1452398-07-0
- Delgrandine
Catalog No.:BCN8122
CAS No.:145237-05-4
- Clobenpropit dihydrobromide
Catalog No.:BCC6781
CAS No.:145231-35-2
- CALP1
Catalog No.:BCC5873
CAS No.:145224-99-3
- Punctanecine
Catalog No.:BCN2018
CAS No.:145204-91-7
- Rizatriptan Benzoate
Catalog No.:BCC3852
CAS No.:145202-66-0
- Kaliotoxin
Catalog No.:BCC7710
CAS No.:145199-73-1
- Iodophenpropit dihydrobromide
Catalog No.:BCC6793
CAS No.:145196-87-8
- 3beta-Hydroxyporiferast-5-en-7-one
Catalog No.:BCN6256
CAS No.:145163-97-9
- CP 99994 dihydrochloride
Catalog No.:BCC6016
CAS No.:145148-39-6
- 6-Hydroxykaempferol 3-Rutinoside -6-glucoside
Catalog No.:BCN1561
CAS No.:145134-63-0
- A 77636 hydrochloride
Catalog No.:BCC7159
CAS No.:145307-34-2
- TC-E 5002
Catalog No.:BCC5608
CAS No.:1453071-47-0
- Bergenin pentaacetate
Catalog No.:BCN6257
CAS No.:14531-47-6
- Dihydropinosylvin
Catalog No.:BCN6258
CAS No.:14531-52-3
- Isochlorogenic acid B
Catalog No.:BCN5909
CAS No.:14534-61-3
- Sideroxylonal A
Catalog No.:BCN1645
CAS No.:145382-68-9
- GDC-0994
Catalog No.:BCC6371
CAS No.:1453848-26-4
- 1,4,7-Eudesmanetriol
Catalog No.:BCN1646
CAS No.:145400-02-8
- Homalomenol A
Catalog No.:BCN1647
CAS No.:145400-03-9
- L-692,585
Catalog No.:BCC7305
CAS No.:145455-35-2
- Heterophyllin B
Catalog No.:BCN2768
CAS No.:145459-19-4
- Isolintetralin
Catalog No.:BCN3052
CAS No.:145459-30-9
Simultaneous determination of five active hydrolysis ingredients from Panax quinquefolium L. by HPLC-ELSD.[Pubmed:20737654]
Biomed Chromatogr. 2011 Jun;25(6):646-51.
An effective method for simultaneous determination of five hydrolysis products of 20 (R)-dammarane-3beta,6alpha,12beta,20,25-pentol, 24(R)-ocotillol, 20(R)-Protopanaxatriol, 20(S)-panaxatriol and 20(R)-dammarane-3beta,12beta,20,25-tetrol was developed using high-performance liquid chromatography with evaporative light scattering detection (HPLC-ELSD). The hydrolysis products from Panax quinquefolium L. in the stems and leaves, berries, flower buds and roots components were successfully separated on a Kromasil C(18) column using methanol and water (83:17, v/v) as mobile phase in 18 min. The parameter for the ELSD was set to a probe temperature of 40 degrees C and the nebulizer for nitrogen gas was adjusted to 3 L/min. All calibration curves showed good linear regression (r > 0.9975) within test ranges. The validation of the method included recovery, linearity, accuracy and precision (intra- and inter-day variation). The accuracy and precision were satisfactory, with the overall intra- and inter-day variation being less than 3.11%, and recoveries of this method were greater than 95.0%. This study developed an effective and rapid method for simultaneous determination of multiple hydrolysis components from Panax quinquefolium L.


