3,4-DihydroxybenzaldehydeCAS# 139-85-5 |
Quality Control & MSDS
Number of papers citing our products

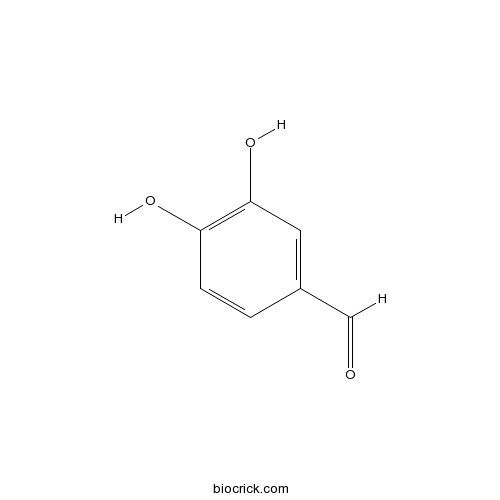
Chemical structure

3D structure
| Cas No. | 139-85-5 | SDF | Download SDF |
| PubChem ID | 8768 | Appearance | Powder |
| Formula | C7H6O3 | M.Wt | 138.1 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (362.00 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 3,4-dihydroxybenzaldehyde | ||
| SMILES | C1=CC(=C(C=C1C=O)O)O | ||
| Standard InChIKey | IBGBGRVKPALMCQ-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3,4-Dihydroxybenzaldehyde, a potent tyrosinase inhibitor, has antifungal activity, it can inhibit oxidative DNA damage and apoptosis via its antioxidant activity. It inhibits the phosphotransferase activity of CKII with IC(50) of about 783 microM, it may function by inhibiting oncogenic disease, at least in part, through the inhibition of CKII activity. It inhibits the H2O2-induced apoptosis of granulosa cells, promotes estradiol secretion in granulosa cells and enhanced the mRNA expression levels of steroidogenic factor 1, a promoter of key steroidogenic enzymes. |
| Targets | ROS | PARP | Antifection | Tyronase | CKII |
| In vitro | 3,4-Dihydroxybenzaldehyde Derived from Prunus mume Seed Inhibits Oxidative Stress and Enhances Estradiol Secretion in Human Ovarian Granulosa Tumor Cells.[Pubmed: 25320407]Acta Histochem Cytochem. 2014 Jun 28;47(3):103-12.Granulosa cells form ovarian follicles and play important roles in the growth and maturation of oocytes. The protection of granulosa cells from cellular injury caused by oxidative stress is an effective therapy for female infertility.
3,4-dihydroxybenzaldehyde, a fungistatic substance from green Cavendish bananas.[Reference: WebLink]Phytochemistry, 1969, 8(2):393-5.A fungistatic substance has been isolated from the outer skin of green Cavendish bananas and identified as 3,4-Dihydroxybenzaldehyde. The compound has been shown to inhibit the growth of Gloeosporium musarum, a fungus which causes ripe fruit rot in the banana. |
| Kinase Assay | Apoptotic cell death through inhibition of protein kinase CKII activity by 3,4-dihydroxybenzaldehyde purified from Xanthium strumarium.[Pubmed: 19023807]Nat Prod Res. 2008;22(16):1441-50.
|
| Cell Research | 3,4-dihydroxybenzaldehyde purified from the barley seeds (Hordeum vulgare) inhibits oxidative DNA damage and apoptosis via its antioxidant activity.[Pubmed: 19022639]Phytomedicine. 2009 Jan;16(1):85-94.Barley is a major crop worldwide. It has been reported that barley seeds have an effect on scavenging ROS. However, little has been known about the functional role of the barley on the inhibition of DNA damage and apoptosis by ROS.
|
| Structure Identification | The Korea Jounnal of Herbology, 2006, 21(2):1-7.Tyronase Inhibitory Effect of 3,4-Dihydroxybenzaldehyde Isolated from Pinellia ternata.[Reference: WebLink]The purpose of this study is to isolate tyrosinase inhibitory material from Pinellia ternata and characterize its own structure and activity.
|

3,4-Dihydroxybenzaldehyde Dilution Calculator

3,4-Dihydroxybenzaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.2411 mL | 36.2056 mL | 72.4113 mL | 144.8226 mL | 181.0282 mL |
| 5 mM | 1.4482 mL | 7.2411 mL | 14.4823 mL | 28.9645 mL | 36.2056 mL |
| 10 mM | 0.7241 mL | 3.6206 mL | 7.2411 mL | 14.4823 mL | 18.1028 mL |
| 50 mM | 0.1448 mL | 0.7241 mL | 1.4482 mL | 2.8965 mL | 3.6206 mL |
| 100 mM | 0.0724 mL | 0.3621 mL | 0.7241 mL | 1.4482 mL | 1.8103 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ziprasidone hydrochloride monohydrate
Catalog No.:BCC2072
CAS No.:138982-67-9
- Capsazepine
Catalog No.:BCC1451
CAS No.:138977-28-3
- Coronarin D ethyl ether
Catalog No.:BCN6203
CAS No.:138965-89-6
- Isocoronarin D
Catalog No.:BCN6202
CAS No.:138965-88-5
- Rutaretin
Catalog No.:BCN4710
CAS No.:13895-92-6
- Ibandronate sodium
Catalog No.:BCC4665
CAS No.:138926-19-9
- Entadamide-A-β-D-glucopyranoside
Catalog No.:BCN8452
CAS No.:138916-58-2
- Brinzolamide
Catalog No.:BCC2313
CAS No.:138890-62-7
- 1-Acetylpiperazine
Catalog No.:BCC8448
CAS No.:13889-98-0
- 5'-Methoxyhexahydrocurcumin
Catalog No.:BCN7049
CAS No.:138870-96-9
- Pasakbumin B
Catalog No.:BCN2991
CAS No.:138809-10-6
- 8'-Oxo-6-hydroxydihydrophaseic acid
Catalog No.:BCN7046
CAS No.:1388075-44-2
- Carmine
Catalog No.:BCN2223
CAS No.:1390-65-4
- Catalpin
Catalog No.:BCN6205
CAS No.:1390-72-3
- Oplodiol
Catalog No.:BCN6204
CAS No.:13902-62-0
- JMV 449
Catalog No.:BCC5863
CAS No.:139026-66-7
- 2-PMDQ
Catalog No.:BCC6726
CAS No.:139047-55-5
- Anemarsaponin B
Catalog No.:BCN6289
CAS No.:139051-27-7
- L-689,560
Catalog No.:BCC6774
CAS No.:139051-78-8
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- (-)-Heraclenol
Catalog No.:BCN7682
CAS No.:139079-42-8
- Tristin
Catalog No.:BCN4709
CAS No.:139101-67-0
- PF 4800567 hydrochloride
Catalog No.:BCC7904
CAS No.:1391052-28-0
- Vigabatrin Hydrochloride
Catalog No.:BCC5198
CAS No.:1391054-02-6
3,4-dihydroxybenzaldehyde purified from the barley seeds (Hordeum vulgare) inhibits oxidative DNA damage and apoptosis via its antioxidant activity.[Pubmed:19022639]
Phytomedicine. 2009 Jan;16(1):85-94.
Barley is a major crop worldwide. It has been reported that barley seeds have an effect on scavenging ROS. However, little has been known about the functional role of the barley on the inhibition of DNA damage and apoptosis by ROS. In this study, we purified 3,4-Dihydroxybenzaldehyde from the barley with silica gel column chromatography and HPLC and then identified it by GC/MS. And we firstly investigated the inhibitory effects of 3,4-Dihydroxybenzaldehyde purified from the barley on oxidative DNA damage and apoptosis induced by H(2)O(2), the major mediator of oxidative stress and a potent mutagen. In antioxidant activity assay such as DPPH radical and hydroxyl radical scavenging assay, Fe(2+) chelating assay, and intracellular ROS scavenging assay by DCF-DA, 3,4-Dihydroxybenzaldehyde was found to scavenge DPPH radical, hydroxyl radical and intracellular ROS. Also it chelated Fe(2+). In in vitro oxidative DNA damage assay and the expression level of phospho-H2A.X, it inhibited oxidative DNA damage and its treatment decreased the expression level of phospho-H2A.X. And in oxidative cell death and apoptosis assay via MTT assay and Hoechst 33342 staining, respectively, the treatment of 3,4-Dihydroxybenzaldehyde attenuated H(2)O(2)-induced cell death and apoptosis. These results suggest that the barley may exert the inhibitory effect on H(2)O(2)-induced tumor development by blocking H(2)O(2)-induced oxidative DNA damage, cell death and apoptosis.
3,4-Dihydroxybenzaldehyde Derived from Prunus mume Seed Inhibits Oxidative Stress and Enhances Estradiol Secretion in Human Ovarian Granulosa Tumor Cells.[Pubmed:25320407]
Acta Histochem Cytochem. 2014 Jun 28;47(3):103-12.
Granulosa cells form ovarian follicles and play important roles in the growth and maturation of oocytes. The protection of granulosa cells from cellular injury caused by oxidative stress is an effective therapy for female infertility. We here investigated an effective bioactive compound derived from Prunus mume seed extract that protects granulosa cells from hydrogen peroxide (H2O2)-induced apoptosis. We detected the bioactive compound, 3,4-Dihydroxybenzaldehyde (3,4-DHBA), via bioactivity-guided isolation and found that it inhibited the H2O2-induced apoptosis of granulosa cells. We also showed that 3,4-DHBA promoted estradiol secretion in granulosa cells and enhanced the mRNA expression levels of steroidogenic factor 1, a promoter of key steroidogenic enzymes. These results suggest that P. mume seed extract may have clinical potential for the prevention and treatment of female infertility.
Preparation and characterization of di-, tri-, and tetranuclear schiff base complexes derived from diamines and 3,4-dihydroxybenzaldehyde.[Pubmed:24453995]
Bioinorg Chem Appl. 2013;2013:219356.
A series of new di-, tri-, and tetranuclear Co(II) and Cu(II) complexes of three new diSchiff base ligands were synthesized by two different methods. The first method involved the synthesis of the three ligands from condensation reaction of 3,4-Dihydroxybenzaldehyde (L'H2) with ethylenediamine (en), o-phenylenediamine (o-PD), or 4,5-dimethyl-1,2-phenylendiamine (DMPD) in a mole ratio of 2 : 1 followed by the reaction of the resulting Schiff bases ligands with Cu(II) or Co(II) ions in the presence of 2,2'-bipyridyl (L) to form the di- and trinuclear metal complexes. The second method involved the condensation of the copper complex LCu(II)L' (L = 2,2'-bipyridyl, L' = 4-formylbenzene-1,2-bis(olate)) with en, o-PD, or DMPD in a mole ratio of 2 : 1, respectively, followed by reaction with CuCl2 or Cu(ClO4)2 to form di-, tri-, and tetranuclear copper (II) complexes, respectively. The structures of the ligands and metal complexes were characterized by elemental analyses, NMR, and FTIR spectra. The geometries of metal complexes were suggested according to elemental analysis, electronic spectra, thermal analyses, atomic absorption, and magnetic moments and conductivity measurements.
Apoptotic cell death through inhibition of protein kinase CKII activity by 3,4-dihydroxybenzaldehyde purified from Xanthium strumarium.[Pubmed:19023807]
Nat Prod Res. 2008;22(16):1441-50.
The CKII inhibitory compound was purified from the fruit of Xanthium strumarium by organic solvent extraction and silica gel chromatography. The inhibitory compound was identified as 3,4-Dihydroxybenzaldehyde by analysis with FT-IR, FAB-Mass, EI-Mass, (1)H-NMR and (13)C-NMR. 3,4-Dihydroxybenzaldehyde inhibited the phosphotransferase activity of CKII with IC(50) of about 783 microM. Steady-state studies revealed that the inhibitor acts as a competitive inhibitor with respect to the substrate ATP. A value of 138.6 microM was obtained for the apparent K(i). Concentration of 300 microM 3,4-Dihydroxybenzaldehyde caused 50% growth inhibition of human cancer cell U937. 3,4-Dihydroxybenzaldehyde-induced cell death was characterised with the cleavage of poly(ADP-ribose) polymerase and procaspase-3. Furthermore, the inhibitor induced the fragmentation of DNA into multiples of 180 bp, indicating that it triggered apoptosis. This induction of apoptosis by 3,4-Dihydroxybenzaldehyde was also confirmed by using flow cytometry analysis. Since CKII is involved in cell proliferation and oncogenesis, these results suggest that 3,4-Dihydroxybenzaldehyde may function by inhibiting oncogenic disease, at least in part, through the inhibition of CKII activity.


