7-HydroxyisoflavoneCAS# 13057-72-2 |
Quality Control & MSDS
Number of papers citing our products

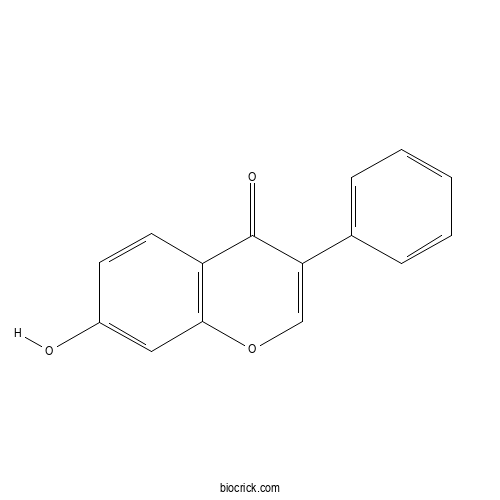
Chemical structure

3D structure
| Cas No. | 13057-72-2 | SDF | Download SDF |
| PubChem ID | 5376891.0 | Appearance | Powder |
| Formula | C15H10O3 | M.Wt | 238.24 |
| Type of Compound | Isoflavones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7-hydroxy-3-phenylchromen-4-one | ||
| SMILES | C1=CC=C(C=C1)C2=COC3=C(C2=O)C=CC(=C3)O | ||
| Standard InChIKey | WMKOZARWBMFKAS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O3/c16-11-6-7-12-14(8-11)18-9-13(15(12)17)10-4-2-1-3-5-10/h1-9,16H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

7-Hydroxyisoflavone Dilution Calculator

7-Hydroxyisoflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1974 mL | 20.9872 mL | 41.9745 mL | 83.949 mL | 104.9362 mL |
| 5 mM | 0.8395 mL | 4.1974 mL | 8.3949 mL | 16.7898 mL | 20.9872 mL |
| 10 mM | 0.4197 mL | 2.0987 mL | 4.1974 mL | 8.3949 mL | 10.4936 mL |
| 50 mM | 0.0839 mL | 0.4197 mL | 0.8395 mL | 1.679 mL | 2.0987 mL |
| 100 mM | 0.042 mL | 0.2099 mL | 0.4197 mL | 0.8395 mL | 1.0494 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Elaidic acid
Catalog No.:BCX1395
CAS No.:112-79-8
- Smilagenin
Catalog No.:BCX1394
CAS No.:126-18-1
- 3-O-Acetylbufotalin
Catalog No.:BCX1393
CAS No.:4029-69-0
- Thevetin A
Catalog No.:BCX1392
CAS No.:37933-66-7
- Peruvoside
Catalog No.:BCX1391
CAS No.:1182-87-2
- Flaccidoside II
Catalog No.:BCX1390
CAS No.:140694-19-5
- Arundoin
Catalog No.:BCX1389
CAS No.:4555-56-0
- γ-Linolenic acid
Catalog No.:BCX1388
CAS No.:506-26-3
- Linoleic acid sodium salt
Catalog No.:BCX1387
CAS No.:822-17-3
- Methyl Eicosapentaenoate
Catalog No.:BCX1386
CAS No.:2734-47-6
- Eicosapentaenoic acid ethyl ester
Catalog No.:BCX1385
CAS No.:86227-47-6
- Docosahexaenoic acid methyl ester
Catalog No.:BCX1384
CAS No.:2566-90-7
- 7α-Hydroxysarsasapogenin
Catalog No.:BCX1397
CAS No.:220832-70-2
- Tapinarof
Catalog No.:BCX1398
CAS No.:79338-84-4
- Senecionine acetate
Catalog No.:BCX1399
CAS No.:126642-77-1
- 4,3',5'-Trihydroxyresveratrol
Catalog No.:BCX1400
CAS No.:637776-83-1
- 7β-Hydroxyganoderenic acid F
Catalog No.:BCX1401
CAS No.:1245946-62-6
- 4-Methoxy-2-[(6-O-β-D-xylopyranosyl-β-D-glucopyranosyl)oxy]benzaldehyde
Catalog No.:BCX1402
CAS No.:140484-68-0
- Gylongiposide I
Catalog No.:BCX1403
CAS No.:206876-12-2
- 2'-Deoxyguanosine monohydrate
Catalog No.:BCX1404
CAS No.:312693-72-4
- Emetine hydrobromide
Catalog No.:BCX1405
CAS No.:52714-87-1
- 1β-Methoxydiversifolin 3-O-methyl ether
Catalog No.:BCX1406
CAS No.:194474-71-0
- 5-Alpha-Hydroxy Laxogenin
Catalog No.:BCX1407
CAS No.:56786-63-1
- Chitohexaose Hexahydrochloride
Catalog No.:BCX1408
CAS No.:127171-88-4
Isolation, Structure Elucidation and In Silico Prediction of Potential Drug-Like Flavonoids from Onosma chitralicum Targeted towards Functionally Important Proteins of Drug-Resistant Bad Bugs.[Pubmed:33918531]
Molecules. 2021 Apr 2;26(7):2048.
Admittedly, the disastrous emergence of drug resistance in prokaryotic and eukaryotic human pathogens has created an urgent need to develop novel chemotherapeutic agents. Onosma chitralicum is a source of traditional medicine with cooling, laxative, and anthelmintic effects. The objective of the current research was to analyze the biological potential of Onosma chitralicum, and to isolate and characterize the chemical constituents of the plant. The crude extracts of the plant prepared with different solvents, such as aqueous, hexane, chloroform(,) ethyl acetate, and butanol, were subjected to antimicrobial activities. Results corroborate that crude (methanol), EtoAc, and n-C(6)H(14) fractions were more active against bacterial strains. Among these fractions, the EtoAc fraction was found more potent. The EtoAc fraction was the most active against the selected microbes, which was subjected to successive column chromatography, and the resultant compounds 1 to 7 were isolated. Different techniques, such as UV, IR, and NMR, were used to characterize the structures of the isolated compounds 1-7. All the isolated pure compounds (1-7) were tested for their antimicrobial potential. Compounds 1 (4',8-dimethoxy-7-Hydroxyisoflavone), 6 (5,3',3-trihydroxy-7,4'-dimethoxyflavanone), and 7 (5',7,8-trihydroxy-6,3',4'-trimethoxyflavanone) were found to be more active against Staphylococcus aureus and Salmonella Typhi. Compound 1 inhibited S. typhi and S. aureus to 10 +/- 0.21 mm and 10 +/- 0.45 mm, whereas compound 6 showed inhibition to 10 +/- 0.77 mm and 9 +/- 0.20 mm, respectively. Compound 7 inhibited S. aureus to 6 +/- 0.36 mm. Compounds 6 and 7 showed significant antibacterial potential, and the structure-activity relationship also justifies their binding to the bacterial enzymes, i.e., beta-hydroxyacyl dehydratase (HadAB complex) and tyrosyl-tRNA synthetase. Both bacterial enzymes are potential drug targets. Further, the isolated compounds were found to be active against the tested fungal strains. Whereas docking identified compound 7, the best binder to the lanosterol 14alpha-demethylase (an essential fungal cell membrane synthesizing enzyme), reported as an antifungal fluconazole binding enzyme. Based on our isolation-linked preliminary structure-activity relationship (SAR) data, we conclude that O. chitralicum can be a good source of natural compounds for drug development against some potential enzyme targets.
Polymorphs and Versatile Solvates of 7-Hydroxyisoflavone.[Pubmed:26935882]
J Pharm Sci. 2016 Apr;105(4):1387-97.
7-Hydroxyisoflavone has been crystallized, identified, and characterized as 2 solvent-free conformational polymorphs and 5 solvates, which differ from each other in the mode of packing and in molecular conformation. All the 7 crystal structures were previously unreported. The conformational polymorphs and solvates were compared by Hirshfeld surface and fingerprint plot analysis and were spectroscopically characterized by powder X-ray diffraction, differential scanning calorimetry, and thermal gravimetric analysis. Hydrogen bond played an important role in the formation of polymorphs. From this study, we can predict that more solvates could be cultivated in other polarity solvents such as isopropanol or 2-butanol at appropriate conditions.
Development of 3-alkyl-6-methoxy-7-hydroxy-chromones (AMHCs) from natural isoflavones, a new class of fluorescent scaffolds for biological imaging.[Pubmed:25429667]
Chem Commun (Camb). 2015 Jan 18;51(5):881-4.
Starting from 7-Hydroxyisoflavones, we developed a new class of fluorescent scaffolds, 3-alkyl-6-methoxy-7-hydroxy-chromones (AMHCs, MW approximately 205.19, lambdaab approximately 350 nm, lambdaem approximately 450 nm) via a trial and error process. AMHCs have the advantages of being a small molecular moiety, having strong fluorescence in basic buffers, reasonable solubility and stability, non-toxicity, and are conveniently linked to pharmacophores. AMHCs were successfully used in fluorescence microscopy imaging of cells and tissues.
The antiviral effect of 7-hydroxyisoflavone against Enterovirus 71 in vitro.[Pubmed:23464760]
J Asian Nat Prod Res. 2013;15(4):382-9.
Enterovirus 71 (EV71) is the major causative agent of hand foot and mouth disease. And EV71 causes epidemics worldwide, particularly in the Asia-Pacific region. Unfortunately, currently there is no approved vaccine or antiviral drug for EV71-induced disease prevention and therapy. In screening for anti-EV71 candidates, we found that 7-Hydroxyisoflavone was active against EV71. 7-Hydroxyisoflavone exhibited strong antiviral activity against three different EV71 strains. The 50% inhibitory concentration range was between 3.25 and 4.92 muM by cytopathic effect assay. 7-Hydroxyisoflavone could reduce EV71 viral RNA and protein synthesis in a dose-dependent manner. Time course study showed that treatment of Vero cells with 7-Hydroxyisoflavone at indicated times after EV71 inoculation (0-6 h) resulted in significant antiviral activity. Results showed that 7-Hydroxyisoflavone acted at an early step of EV71 replication. 7-Hydroxyisoflavone also exhibited strong antiviral activity against coxsackievirus B2, B3, and B6. In short, 7-Hydroxyisoflavone may be used as a lead compound for anti-EV71 drug development.
Stereospecific microbial production of isoflavanones from isoflavones and isoflavone glucosides.[Pubmed:21562980]
Appl Microbiol Biotechnol. 2011 Aug;91(4):1173-81.
A Gram-negative anaerobic microorganism, MRG-1, isolated from human intestine showed high activities of deglycosylation and reduction of daidzin, based on rapid TLC analysis. A rod-shaped strain MRG-1 was identified as a new species showing 91.0% homology to Coprobacillus species, based on 16S rRNA sequence analysis. The strain MRG-1 showed beta-glucosidase activity toward daidzin and genistin, and daidzein and genistein were produced, respectively. However, the strain MRG-1 did not react with flavone glycosides, flavanone glycosides, and isoflavone C-glucoside. Besides, MRG-1 showed stereoselective reductase activity to isoflavone, daidzein, genistein, 7-Hydroxyisoflavone, and formononetin, resulting in the formation of corresponding R-isoflavanone enantiomers. The new isoflavanones of 7-hydroxyisoflavanone and dihydroformononetin were characterized by NMR, and the absolute configurations of the enantiomers were determined with CD spectroscopy. The kinetic study of the anaerobic biotransformation showed both activities were exceptionally fast compared to the reported conversion by other anaerobic bacteria.
[Chemical constituents of bear bile].[Pubmed:21141490]
Zhongguo Zhong Yao Za Zhi. 2010 Sep;35(18):2416-9.
OBJECTIVE: To study the chemical constituents of bear bile. METHOD: The compounds were isolated by repeated column HP20 macroporous adsorption resin, Sephadex LH-20, ODS and silica gel as packing materials. The structures were identified on the basis of extensive spectroscopic data analysis and by comparison of their spectral data reported. RESULT: Nine compounds were identified as 4',7-dihydroxyisoflavone (1), 4',7-dihydroxy-6-methoxyisoflavone (2), 4',6,7-trihydroxyisoflavone (3), 4'-methoxy-7-Hydroxyisoflavone (4), tauroursodeoxycholic acid (5), taurochenodeoxycholic acid (6), ursodeoxycholic acid (7), chenodeoxycholic acid (8), cholesterol (9). CONCLUSION: Compounds 1-4 were separated from bear bile for the first time.
Bioactive isoflavones from Dalbergia vacciniifolia (Fabaceae).[Pubmed:20614820]
Nat Prod Commun. 2010 Jun;5(6):903-6.
Three new 5-dehydroxy isoflavone compounds, 6,2'-dimethoxy-7,4'-dihydroxyisoflavone (1), 6,2',4'-trimethoxy-7-Hydroxyisoflavone (2), and 6,2',4',5'-tetramethoxy-7-O-[beta-D-apiofuranosyl-(1 --> 6)-beta-D-glucopyranoside] isoflavone (3), along with a known isoflavone, 6,2',4',5'-tetramethoxy-7-Hydroxyisoflavone (4), were isolated from the ethanolic extract of Dalbergia vacciniifolia Vatke. Their structures were established by spectroscopic techniques including one- and two-dimension NMR. Compound 1 showed mild cytotoxic activity against brine shrimp larvae with a LC50 value of 266 microg/mL.
Flavonoids from Echinops echinatus.[Pubmed:16864424]
J Asian Nat Prod Res. 2006 Apr-May;8(3):197-200.
A new isoflavone glycoside, echinoside (7), together with 7-Hydroxyisoflavone, kaempferol-4'-methylether, kaempferol-7-methylether, myrecetin-3-O-alpha-L-rhamnoside, kaempferol and kaempferol-3-O-alpha-L-rhamnoside, has been isolated from the whole plant of Echinops echinatus. The structure of echinoside has been established by chemical and spectral data. This is the first report of the occurrence of these flavonoids in E. echinatus.
Biotransformation of natural and synthetic isoflavonoids by two recombinant microbial enzymes.[Pubmed:12957885]
Appl Environ Microbiol. 2003 Sep;69(9):5045-50.
Isolation and synthesis of isoflavonoids has become a frequent endeavor, due to their interesting biological activities. The introduction of hydroxyl groups into isoflavonoids by the use of enzymes represents an attractive alternative to conventional chemical synthesis. In this study, the capabilities of biphenyl-2,3-dioxygenase (BphA) and biphenyl-2,3-dihydrodiol 2,3-dehydrogenase (BphB) of Burkholderia sp. strain LB400 to biotransform 14 isoflavonoids synthesized in the laboratory were investigated by using recombinant Escherichia coli strains containing plasmid vectors expressing the bphA1A2A3A4 or bphA1A2A3A4B genes of strain LB400. The use of BphA and BphB allowed us to biotransform 7-hydroxy-8-methylisoflavone and 7-Hydroxyisoflavone into 7,2',3'-trihydroxy-8-methylisoflavone and 7,3',4'-trihydroxyisoflavone, respectively. The compound 2'-fluoro-7-hydroxy-8-methylisoflavone was dihydroxylated by BphA at ortho-fluorinated and meta positions of ring B, with concomitant dehalogenation leading to 7,2',3',-trihydroxy-8-methylisoflavone. Daidzein (7,4'-dihydroxyisoflavone) was biotransformed by BphA, generating 7,2',4'-trihydroxyisoflavone after dehydration. Biotransformation products were analyzed by gas chromatography-mass spectrometry and nuclear magnetic resonance techniques.
Activity-guided isolation of the chemical constituents of Muntingia calabura using a quinone reductase induction assay.[Pubmed:12737982]
Phytochemistry. 2003 Jun;63(3):335-41.
Activity-guided fractionation of an EtOAc-soluble extract of the leaves of Muntingia calabura collected in Peru, using an in vitro quinone reductase induction assay with cultured Hepa 1c1c7 (mouse hepatoma) cells, resulted in the isolation of a flavanone with an unsubstituted B-ring, (2R,3R)-7-methoxy-3,5,8-trihydroxyflavanone (5), as well as 24 known compounds, which were mainly flavanones and flavones. The structure including absolute stereochemistry of compound 5 was determined by spectroscopic (HRMS, 1D and 2D NMR, and CD spectra) methods. Of the isolates obtained, in addition to 5, (2S)-5-hydroxy-7-methoxyflavanone, 2',4'-dihydroxychalcone, 4,2',4'-trihydroxychalcone, 7-Hydroxyisoflavone and 7,3',4'-trimethoxyisoflavone were found to induce quinone reductase activity.


