9,13-Epidioxy-8(14)-abieten-18-oic acidCAS# 5309-35-3 |
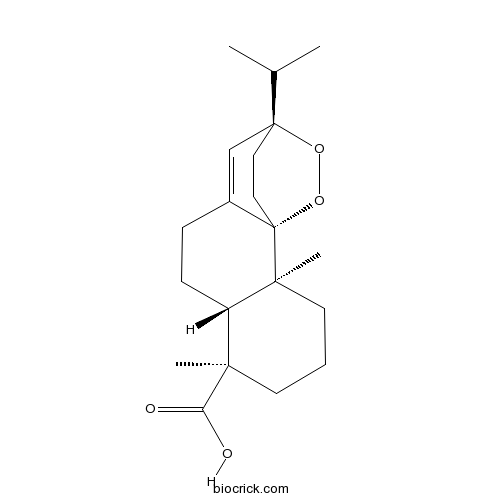
- 9alpha,13alpha-Epidioxyabiet-8(14)-en-18-oic acid
Catalog No.:BCN1611
CAS No.:116499-73-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 5309-35-3 | SDF | Download SDF |
| PubChem ID | 46230362 | Appearance | Powder |
| Formula | C20H30O4 | M.Wt | 334.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,2S,6R,7R,12S)-2,6-dimethyl-12-propan-2-yl-13,14-dioxatetracyclo[10.2.2.01,10.02,7]hexadec-10-ene-6-carboxylic acid | ||
| SMILES | CC(C)C12CCC3(C(=C1)CCC4C3(CCCC4(C)C(=O)O)C)OO2 | ||
| Standard InChIKey | OOHFJWCHEFCJDC-ZMHJYQQXSA-N | ||
| Standard InChI | InChI=1S/C20H30O4/c1-13(2)19-10-11-20(24-23-19)14(12-19)6-7-15-17(3,16(21)22)8-5-9-18(15,20)4/h12-13,15H,5-11H2,1-4H3,(H,21,22)/t15-,17+,18-,19+,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 9,13β-Epidioxy-8(14)-abieten-18-oic acid exhibits moderate activities on NO levels in LPS-stimulated murine microglia BV2 cells, with IC50 values of 57.3 ± 0.2 uM, suggests that it has anti-inflammatory activities. 2. 9α,13α-Epidioxyabiet-8(14)-en-18-oic acid shows potent inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) activation induced by the tumor promoter 12-O-tetradecanoylphorbol 13-acetate, suggests that it is a potential antitumor-promoting diterpenoid. 3. 9 α ,13 β -Epidioxyabeit-8(14)en-18-oic acid may contribute to the growth inhibitory effect of the pine needles and may play an important role in the allelopathy of red pine. |
| Targets | NO |

9,13-Epidioxy-8(14)-abieten-18-oic acid Dilution Calculator

9,13-Epidioxy-8(14)-abieten-18-oic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9895 mL | 14.9477 mL | 29.8954 mL | 59.7907 mL | 74.7384 mL |
| 5 mM | 0.5979 mL | 2.9895 mL | 5.9791 mL | 11.9581 mL | 14.9477 mL |
| 10 mM | 0.299 mL | 1.4948 mL | 2.9895 mL | 5.9791 mL | 7.4738 mL |
| 50 mM | 0.0598 mL | 0.299 mL | 0.5979 mL | 1.1958 mL | 1.4948 mL |
| 100 mM | 0.0299 mL | 0.1495 mL | 0.299 mL | 0.5979 mL | 0.7474 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Morellic acid
Catalog No.:BCN3073
CAS No.:5304-71-2
- Scutebarbatine J
Catalog No.:BCN8134
CAS No.:960302-85-6
- T-5224
Catalog No.:BCC5383
CAS No.:530141-72-1
- Murralongin
Catalog No.:BCN5696
CAS No.:53011-72-6
- Salinomycin
Catalog No.:BCC1916
CAS No.:53003-10-4
- CDI (1,1′-Carbonyldiimidazole)
Catalog No.:BCC2809
CAS No.:530-62-1
- Sinapic acid
Catalog No.:BCN3539
CAS No.:530-59-6
- Syringic acid
Catalog No.:BCN5699
CAS No.:530-57-4
- 2,6-Dimethoxy-1,4-benzoquinone
Catalog No.:BCN5698
CAS No.:530-55-2
- Deoxyvasicinone
Catalog No.:BCN5697
CAS No.:530-53-0
- L-Picein
Catalog No.:BCC8336
CAS No.:530-14-3
- Indomethacin
Catalog No.:BCC3794
CAS No.:53-86-1
- Dichotomin
Catalog No.:BCN2836
CAS No.:53093-47-3
- Androsin
Catalog No.:BCN3842
CAS No.:531-28-2
- Coniferin
Catalog No.:BCN5700
CAS No.:531-29-3
- Scopolin
Catalog No.:BCN5701
CAS No.:531-44-2
- 7-Methoxycoumarin
Catalog No.:BCN2707
CAS No.:531-59-9
- Esculin
Catalog No.:BCN5904
CAS No.:531-75-9
- Coumarin-3-Carboxylic Acid
Catalog No.:BCC9220
CAS No.:531-81-7
- 4',7-Isoflavandiol
Catalog No.:BCN2855
CAS No.:531-95-3
- Boc-Pyr-OH
Catalog No.:BCC3329
CAS No.:53100-44-0
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
- Buprenorphine hydrochloride
Catalog No.:BCC5215
CAS No.:53152-21-9
- Euscaphic acid
Catalog No.:BCN5702
CAS No.:53155-25-2
An allelopathic substance in red pine needles (Pinus densiflora).[Pubmed:18755523]
J Plant Physiol. 2009 Mar 1;166(4):442-6.
Aqueous methanol extracts of red pine (Pinus densiflora) needles inhibited the growth of roots and shoots of cress (Lepidium sativum), lettuce (Lactuca sativa), alfalfa (Medicago sativa), ryegrass (Lolium multiflorum), timothy (Pheleum pratense), Digitaria sanguinalis and Echinochloa crus-galli. Increasing the extract concentration increased inhibition, suggesting that the pine needles may have growth inhibitory substances and possess allelopathic potential. The aqueous methanol extract of the pine needles was purified, and a main inhibitory substance was isolated and determined by spectral data as 9alpha,13beta-epidioxyabeit-8(14)en-18-oic acid. This substance inhibited root and shoot growth of cress and Echinochloa crus-galli seedlings at concentrations greater than 0.1 mM. The endogenous concentration of the substance was 0.13 mmol/kg pine needle. These results suggest that 9alpha,13beta-epidioxyabeit-8(14)en-18-oic acid may contribute to the growth inhibitory effect of the pine needles and may play an important role in the allelopathy of red pine.
Potential antitumor-promoting diterpenoids from the stem bark of Picea glehni.[Pubmed:10869208]
J Nat Prod. 2000 Jun;63(6):817-20.
A novel rearranged labdane-type diterpenoid, 19(4-->3)abeo-8alpha, 13(S)-epoxylabda-4(18),14-diene (1), and two new abietane-type diterpenoids, 19-nor-abieta-4(18),8,11,13-tetraen-7-one (2) and 12-hydroxydehydroabietic acid (3) were isolated from the stem bark of Picea glehni, together with seven known diterpenoids-13-epimanoyl oxide (4), dehydroabietic acid (5), (11E)-14, 15-bisnor-8alpha-hydroxy-11-labden-13-one (6), abieta-8,11, 13-trien-7-one (7), 9alpha,13alpha-epidioxyabiet-8(14)-en-18-oic acid (8), 9,10alpha-epoxy-9,10-seco-abieta-8,11,13-trien-18-oic acid (9), and methyl 15-hydroxy-7-oxo-dehydroabietate (10). Compounds 5-8 and 10 showed potent inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) activation induced by the tumor promoter 12-O-tetradecanoylphorbol 13-acetate.
Diterpenes from the Trunk of Abies holophylla and Their Potential Neuroprotective and Anti-inflammatory Activities.[Pubmed:26812172]
J Nat Prod. 2016 Feb 26;79(2):387-94.
Eleven new abietane-type diterpenes, holophyllins D-N (1-11), and 17 known analogues (12-28), were isolated from a MeOH extract of the trunk of Abies holophylla. The chemical structures of 1-11 were determined through spectroscopic data analysis, including NMR ((1)H and (13)C NMR, DEPT, (1)H-(1)H COSY, HMQC, HMBC, and NOESY) and HRFABMS methods. All isolated compounds (1-28) were evaluated for their cytotoxicity against four human tumor cell lines (A549, SK-OV-3, SK-MEL-2, and HCT-116), for their potential neuroprotective effects through induction of nerve growth factor in C6 glioma cells, and for their effects on nitric oxide levels in lipopolysaccharide-stimulated murine microglia BV2 cells.


