A 943931 dihydrochlorideAntagonist of histamine H4 receptor,potent and selective CAS# 1227675-50-4 |
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- SD-208
Catalog No.:BCC1938
CAS No.:627536-09-8
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
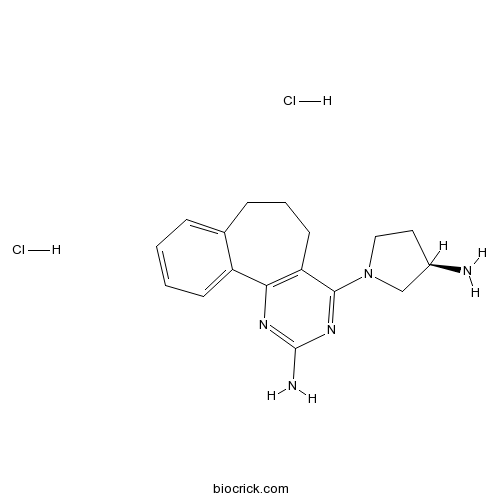
| Cas No. | 1227675-50-4 | SDF | Download SDF |
| PubChem ID | 56972240 | Appearance | Powder |
| Formula | C17H23Cl2N5 | M.Wt | 368.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
| Chemical Name | 4-[(3R)-3-aminopyrrolidin-1-yl]-6,7-dihydro-5H-benzo[1,2]cyclohepta[3,4-b]pyrimidin-2-amine;dihydrochloride | ||
| SMILES | C1CC2=CC=CC=C2C3=C(C1)C(=NC(=N3)N)N4CCC(C4)N.Cl.Cl | ||
| Standard InChIKey | ISYUFRNZHUOGLA-CURYUGHLSA-N | ||
| Standard InChI | InChI=1S/C17H21N5.2ClH/c18-12-8-9-22(10-12)16-14-7-3-5-11-4-1-2-6-13(11)15(14)20-17(19)21-16;;/h1-2,4,6,12H,3,5,7-10,18H2,(H2,19,20,21);2*1H/t12-;;/m1../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective histamine H4 receptor antagonist (pKi values are 7.15 and 8.12 at human and rat receptors respectively). Blocks inflammation in a peritonitis mouse model and displays efficacy in inflammatory pain and neuropathic pain models. |

A 943931 dihydrochloride Dilution Calculator

A 943931 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7151 mL | 13.5755 mL | 27.151 mL | 54.3021 mL | 67.8776 mL |
| 5 mM | 0.543 mL | 2.7151 mL | 5.4302 mL | 10.8604 mL | 13.5755 mL |
| 10 mM | 0.2715 mL | 1.3576 mL | 2.7151 mL | 5.4302 mL | 6.7878 mL |
| 50 mM | 0.0543 mL | 0.2715 mL | 0.543 mL | 1.086 mL | 1.3576 mL |
| 100 mM | 0.0272 mL | 0.1358 mL | 0.2715 mL | 0.543 mL | 0.6788 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A 943931, is an H4R (one of histamine receptor subtypes) antagonist [1] with high affinities to H4Rs of human (Ki = 5 nM), rat (Ki = 4 nM) and mouse (Kb = 6 nM) [2].
H4R is one of 4 known G-protein-coupled receptors (H1, H2, H3 and H4 receptors) of histamine for histamine to mediate its physiological functions [3].
HMC-1 cells incubated with A 943931 at a concentration of 300 nM for 20 min inhibited the increase in ALDH2 activity induced by H4R [4]. In microglia, A 943931 at a concentration of 10 μM partially abolish the release of TNF-α and IL-6 induced by histamine at a concentration of 0.1 μg/ml [5]. In bone marrow-derived mast cells, A 943931 inhibited the shape change induced by histamine (IC50 = 0.38 μM) [6].
Intraperitoneal administration of A 943931 at a dose of 33 μmol/kg potently inhibited itch induced by H4R agonist in mice [6]. In several preclinical models, H4R had been shown to be linked to inflammation [7]. A 943931 had excellent antagonistic activity both in vivo and in vitro across multiple species, displayed good oral bioavailability (90%) and excellent metabolic stability. This compound displays good efficacy in rat pain models and is a good anti-inflammatory agent in mice [8]. A 943931 has an in vivo oral bioavailability of 34% and a half-life of 2.6 h in rats [2]. A 943931 efficaciously reduced acute inflammatory pains induced by formalin in the flinch model and by carrageenan in mechanical and thermal hyperalgesia models in rats [9].
References:
[1]. Erich H. Schneider and Roland Seifert. The histamine H4-receptor and the central and peripheral nervous system: A critical analysis of the literature. Neuropharmacology, 2015, xxx:1-13.
[2]. Rogier A. Smits, Herman D. Lim, Tiffany van der Meer, et al. Ligand based design of novel histamine H4 receptor antagonists; fragment optimization and analysis of binding kinetics. Bioorg. Med. Chem. Lett., 2012, 22: 461-467.
[3]. Huaqing Liu, Robert J. Altenbach, Tracy L. Carr, et al. cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2-amine (A-987306), A New Histamine H4R Antagonist that Blocks Pain Responses against Carrageenan-Induced Hyperalgesia. J. Med. Chem., 2008, 51:7094-7098.
[4]. Silvia Aldi, Ken-ichi Takano, Kengo Tomita, et al. Histamine H4-Receptors Inhibit Mast Cell Renin Release in Ischemia/Reperfusion via PKCε-Dependent Aldehyde Dehydrogenase Type-2 Activation. J. Pharmacol. Exp. Ther., 2014, 349(3):508-17.
[5]. Jin Zhu, Chen Qu, Xiang Lu, et al. Activation of Microglia by Histamine and Substance P. Cell Physiol. Biochem., 2014, 34(3):768-80.
[6]. Harald Engelhardt, Rogier A Smits, Rob Leurs, et al. A new generation of anti-histamines: Histamine H4 receptor antagonists on their way to the clinic. Curr. Opin. Drug Discov. Devel., 2009, 12(5):628-43.
[7]. Jeffery M Cowden, Fuqu Yu, Homayon Banie, et al. The histamine H4 receptor mediates inflammation and Th17 responses in preclinical models of arthritis. Ann. Rheum. Dis., 2014, 73:600-608.
[8]. Rob Leurs, Paul L Chazot, Fiona C Shenton, et al. Molecular and biochemical pharmacology of the histamine H4 receptor. British Journal of Pharmacology, 2009, 157: 14-23.
[9]. David Burns, Niu Shin, Ravi Jalluri, et al. Annual Reports in Medicinal Chemistry: H4 Receptor Antagonists and Their Potential Therapeutic Applications. Burlington: Academic Press, 2014.
- StemRegenin 1 (SR1)
Catalog No.:BCC3637
CAS No.:1227633-49-9
- [D-Ala2]-Deltorphin II
Catalog No.:BCC5723
CAS No.:122752-16-3
- Deltorphin I
Catalog No.:BCC6233
CAS No.:122752-15-2
- Bi-linderone
Catalog No.:BCN6116
CAS No.:1227375-09-8
- Philanthotoxin 74
Catalog No.:BCC7478
CAS No.:1227301-51-0
- Liangshanin A
Catalog No.:BCN6115
CAS No.:122717-54-8
- AZD3839
Catalog No.:BCC6471
CAS No.:1227163-84-9
- 4-Fluoro-1-(3-(pyrimidin-5-yl)phenyl)-1-(2-(trifluoromethyl)pyridin-4-yl)-1H-isoindol-3-amine
Catalog No.:BCC5113
CAS No.:1227163-56-5
- BAY 87-2243
Catalog No.:BCC4131
CAS No.:1227158-85-1
- SB 277011A dihydrochloride
Catalog No.:BCC7887
CAS No.:1226917-67-4
- ATB-346
Catalog No.:BCC5289
CAS No.:1226895-20-0
- FLLL32
Catalog No.:BCC6499
CAS No.:1226895-15-3
- SCH 39166 hydrobromide
Catalog No.:BCC7317
CAS No.:1227675-51-5
- MNI-caged-NMDA
Catalog No.:BCC5888
CAS No.:1227675-52-6
- CEP-32496 hydrochloride
Catalog No.:BCC1468
CAS No.:1227678-26-3
- ACTH (1-39)
Catalog No.:BCC6028
CAS No.:12279-41-3
- GSK2334470
Catalog No.:BCC4982
CAS No.:1227911-45-6
- Gadodiamide
Catalog No.:BCC4663
CAS No.:122795-43-1
- Marinopyrrole A
Catalog No.:BCC4098
CAS No.:1227962-62-0
- Sabutoclax
Catalog No.:BCC2236
CAS No.:1228108-65-3
- 8-Geranyloxy-5,7-dimethoxycoumarin
Catalog No.:BCN6117
CAS No.:1228175-65-2
- MRS 2957 triethylammonium salt
Catalog No.:BCC6133
CAS No.:1228271-30-4
- H-D-Phe(4-F)-OH .HCl
Catalog No.:BCC3217
CAS No.:122839-52-5
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
Investigating antimalarial drug interactions of emetine dihydrochloride hydrate using CalcuSyn-based interactivity calculations.[Pubmed:28257497]
PLoS One. 2017 Mar 3;12(3):e0173303.
The widespread introduction of artemisinin-based combination therapy has contributed to recent reductions in malaria mortality. Combination therapies have a range of advantages, including synergism, toxicity reduction, and delaying the onset of resistance acquisition. Unfortunately, antimalarial combination therapy is limited by the depleting repertoire of effective drugs with distinct target pathways. To fast-track antimalarial drug discovery, we have previously employed drug-repositioning to identify the anti-amoebic drug, emetine dihydrochloride hydrate, as a potential candidate for repositioned use against malaria. Despite its 1000-fold increase in in vitro antimalarial potency (ED50 47 nM) compared with its anti-amoebic potency (ED50 26-32 uM), practical use of the compound has been limited by dose-dependent toxicity (emesis and cardiotoxicity). Identification of a synergistic partner drug would present an opportunity for dose-reduction, thus increasing the therapeutic window. The lack of reliable and standardised methodology to enable the in vitro definition of synergistic potential for antimalarials is a major drawback. Here we use isobologram and combination-index data generated by CalcuSyn software analyses (Biosoft v2.1) to define drug interactivity in an objective, automated manner. The method, based on the median effect principle proposed by Chou and Talalay, was initially validated for antimalarial application using the known synergistic combination (atovaquone-proguanil). The combination was used to further understand the relationship between SYBR Green viability and cytocidal versus cytostatic effects of drugs at higher levels of inhibition. We report here the use of the optimised Chou Talalay method to define synergistic antimalarial drug interactivity between emetine dihydrochloride hydrate and atovaquone. The novel findings present a potential route to harness the nanomolar antimalarial efficacy of this affordable natural product.
Original research paper. Characterization and taste masking evaluation of microparticles with cetirizine dihydrochloride and methacrylate-based copolymer obtained by spray drying.[Pubmed:28231047]
Acta Pharm. 2017 Mar 1;67(1):113-124.
Taste of a pharmaceutical formulation is an important parameter for the effectiveness of pharmacotherapy. Cetirizine dihydrochloride (CET) is a second-generation antihistamine that is commonly administered in allergy treatment. CET is characterized by extremely bitter taste and it is a great challenge to successfully mask its taste; therefore the goal of this work was to formulate and characterize the microparticles obtained by the spray drying method with CET and poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate 1:2:1 copolymer (Eudragit E PO) as a barrier coating. Assessment of taste masking by the electronic tongue has revealed that designed formulations created an effective taste masking barrier. Taste masking effect was also confirmed by the in vivo model and the in vitro release profile of CET. Obtained data have shown that microparticles with a drug/polymer ratio (0.5:1) are promising CET carriers with efficient taste masking potential and might be further used in designing orodispersible dosage forms with CET.
Pretreatment cognitive and neural differences between sapropterin dihydrochloride responders and non-responders with phenylketonuria.[Pubmed:28271047]
Mol Genet Metab Rep. 2017 Feb 23;12:8-13.
Sapropterin dihydrochloride (BH4) reduces phenylalanine (Phe) levels and improves white matter integrity in a subset of individuals with phenylketonuria (PKU) known as "responders." Although prior research has identified biochemical and genotypic differences between BH4 responders and non-responders, cognitive and neural differences remain largely unexplored. To this end, we compared intelligence and white matter integrity prior to treatment with BH4 in 13 subsequent BH4 responders with PKU, 16 subsequent BH4 non-responders with PKU, and 12 healthy controls. Results indicated poorer intelligence and white matter integrity in non-responders compared to responders prior to treatment. In addition, poorer white matter integrity was associated with greater variability in Phe across the lifetime in non-responders but not in responders. These results underscore the importance of considering PKU as a multi-faceted, multi-dimensional disorder and point to the need for additional research to delineate characteristics that predict response to treatment with BH4.
Proposed phase 2/ step 2 in-vitro test on basis of EN 14561 for standardised testing of the wound antiseptics PVP-iodine, chlorhexidine digluconate, polihexanide and octenidine dihydrochloride.[Pubmed:28193164]
BMC Infect Dis. 2017 Feb 13;17(1):143.
BACKGROUND: Currently, there is no agreed standard for exploring the antimicrobial activity of wound antiseptics in a phase 2/ step 2 test protocol. In the present study, a standardised in-vitro test is proposed, which allows to test potential antiseptics in a more realistically simulation of conditions found in wounds as in a suspension test. Furthermore, factors potentially influencing test results such as type of materials used as test carrier or various compositions of organic soil challenge were investigated in detail. METHODS: This proposed phase 2/ step 2 test method was modified on basis of the EN 14561 by drying the microbial test suspension on a metal carrier for 1 h, overlaying the test wound antiseptic, washing-off, neutralization, and dispersion at serial dilutions at the end of the required exposure time yielded reproducible, consistent test results. RESULTS: The difference between the rapid onset of the antiseptic effect of PVP-I and the delayed onset especially of polihexanide was apparent. Among surface-active antimicrobial compounds, octenidine was more effective than chlorhexidine digluconate and polihexanide, with some differences depending on the test organisms. However, octenidine and PVP-I were approximately equivalent in efficiency and microbial spectrum, while polihexanide required longer exposure times or higher concentrations for a comparable antimicrobial efficacy. CONCLUSION: Overall, this method allowed testing and comparing differ liquid and gel based antimicrobial compounds in a standardised setting.
Histamine H4-receptors inhibit mast cell renin release in ischemia/reperfusion via protein kinase C epsilon-dependent aldehyde dehydrogenase type-2 activation.[Pubmed:24696042]
J Pharmacol Exp Ther. 2014 Jun;349(3):508-17.
Renin released by ischemia/reperfusion (I/R) from cardiac mast cells (MCs) activates a local renin-angiotensin system (RAS) causing arrhythmic dysfunction. Ischemic preconditioning (IPC) inhibits MC renin release and consequent activation of this local RAS. We postulated that MC histamine H4-receptors (H4Rs), being Galphai/o-coupled, might activate a protein kinase C isotype-epsilon (PKCepsilon)-aldehyde dehydrogenase type-2 (ALDH2) cascade, ultimately eliminating MC-degranulating and renin-releasing effects of aldehydes formed in I/R and associated arrhythmias. We tested this hypothesis in ex vivo hearts, human mastocytoma cells, and bone marrow-derived MCs from wild-type and H4R knockout mice. We found that activation of MC H4Rs mimics the cardioprotective anti-RAS effects of IPC and that protection depends on the sequential activation of PKCepsilon and ALDH2 in MCs, reducing aldehyde-induced MC degranulation and renin release and alleviating reperfusion arrhythmias. These cardioprotective effects are mimicked by selective H4R agonists and disappear when H4Rs are pharmacologically blocked or genetically deleted. Our results uncover a novel cardioprotective pathway in I/R, whereby activation of H4Rs on the MC membrane, possibly by MC-derived histamine, leads sequentially to PKCepsilon and ALDH2 activation, reduction of toxic aldehyde-induced MC renin release, prevention of RAS activation, reduction of norepinephrine release, and ultimately to alleviation of reperfusion arrhythmias. This newly discovered protective pathway suggests that MC H4Rs may represent a new pharmacologic and therapeutic target for the direct alleviation of RAS-induced cardiac dysfunctions, including ischemic heart disease and congestive heart failure.
Molecular and biochemical pharmacology of the histamine H4 receptor.[Pubmed:19413568]
Br J Pharmacol. 2009 May;157(1):14-23.
The elucidation of the human genome has had a major impact on histamine receptor research. The identification of the human H4 receptor by several groups has been instrumental for a new appreciation of the role of histamine in the modulation of immune function. In this review, we summarize the historical developments and the molecular and biochemical pharmacology of the H4 receptor.
Rotationally constrained 2,4-diamino-5,6-disubstituted pyrimidines: a new class of histamine H4 receptor antagonists with improved druglikeness and in vivo efficacy in pain and inflammation models.[Pubmed:18817367]
J Med Chem. 2008 Oct 23;51(20):6547-57.
A new structural class of histamine H 4 receptor antagonists (6-14) was designed based on rotationally restricted 2,4-diaminopyrimidines. Series compounds showed potent and selective in vitro H 4 antagonism across multiple species, good CNS penetration, improved PK properties compared to reference H 4 antagonists, functional H 4 antagonism in cellular and in vivo pharmacological assays, and in vivo anti-inflammatory and antinociceptive efficacy. One compound, 10 (A-943931), combined the best features of the series in a single molecule and is an excellent tool compound to probe H 4 pharmacology. It is a potent H 4 antagonist in functional assays across species (FLIPR Ca (2+) flux, K b < 5.7 nM), has high (>190x) selectivity for H 4, and combines good PK in rats and mice (t 1/2 of 2.6 and 1.6 h, oral bioavailability of 37% and 90%) with anti-inflammatory activity (ED 50 = 37 micromol/kg, mouse) and efficacy in pain models (thermal hyperalgesia, ED 50 = 72 micromol/kg, rat).


