AbacavirInhibitor of HIV reverse transcriptase CAS# 136470-78-5 |
- FT-207 (NSC 148958)
Catalog No.:BCC4455
CAS No.:17902-23-7
- Orotic acid
Catalog No.:BCC4162
CAS No.:65-86-1
- Adenine
Catalog No.:BCC4450
CAS No.:73-24-5
- Leflunomide
Catalog No.:BCC1256
CAS No.:75706-12-6
- LY2334737
Catalog No.:BCC4060
CAS No.:892128-60-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 136470-78-5 | SDF | Download SDF |
| PubChem ID | 441300 | Appearance | Powder |
| Formula | C14H18N6O | M.Wt | 286.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (349.25 mM; Need ultrasonic) H2O : 2 mg/mL (6.98 mM; Need ultrasonic) | ||
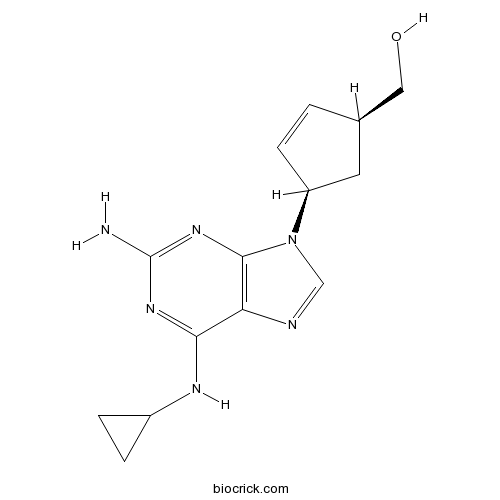
| Chemical Name | [(1S,4R)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl]methanol | ||
| SMILES | C1CC1NC2=NC(=NC3=C2N=CN3C4CC(C=C4)CO)N | ||
| Standard InChIKey | MCGSCOLBFJQGHM-SCZZXKLOSA-N | ||
| Standard InChI | InChI=1S/C14H18N6O/c15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21/h1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19)/t8-,10+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Abacavir is a powerful nucleoside analog reverse transcriptase inhibitor (NRTI) used to treat HIV and AIDS.
IC50 value:
Target: NRTI; reverse transcriptase inhibitor
Abacavir is a nucleoside reverse transcriptase inhibitor marketed since 1999 for the treatment of infection with the human immunodeficiency virus type 1 (HIV). Despite its clinical efficacy, abacavir administration has been associated with serious and sometimes fatal toxic events. Abacavir has been reported to undergo bioactivation in vitro, yielding reactive species that bind covalently to human serum albumin, but the haptenation mechanism and its significance to the toxic events induced by this anti-HIV drug have yet to be elucidated.
The mechanism underlying abacavir hypersensitivity syndrome is related to the change in the HLA-B*5701 protein product. Abacavir binds with high specificity to the HLA-B*5701 protein, changing the shape and chemistry of the antigen-binding cleft. This results in a change in immunological tolerance and the subsequent activation of abacavir-specific cytotoxic T cells, which produce a systemic reaction known as abacavir hypersensitivity syndrome. References: | |||||

Abacavir Dilution Calculator

Abacavir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4925 mL | 17.4624 mL | 34.9247 mL | 69.8495 mL | 87.3118 mL |
| 5 mM | 0.6985 mL | 3.4925 mL | 6.9849 mL | 13.9699 mL | 17.4624 mL |
| 10 mM | 0.3492 mL | 1.7462 mL | 3.4925 mL | 6.9849 mL | 8.7312 mL |
| 50 mM | 0.0698 mL | 0.3492 mL | 0.6985 mL | 1.397 mL | 1.7462 mL |
| 100 mM | 0.0349 mL | 0.1746 mL | 0.3492 mL | 0.6985 mL | 0.8731 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50 Value: 0.26 microM for HIV-1[1] Abacavir,(-)-(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol, is a novel purine carbocyclic nucleoside analogue that has been approved by the FDA for the treatment of HIV (as Ziagen trade mark [abacavir sulfate]) [2]. in vitro: In erythrocytes, abacavir influx was rapid, nonsaturable (rate constant=200 pmol/s/mM/microl cell water), and unaffected by inhibitors of nucleoside or nucleobase transport[2]. in vivo: pharmacokinetic, distribution, and toxicological profiles of 1592U89 were distinct from and improved over those of CBV, probably because CBV itself was not appreciably formed from 1592U89 in cells or animals (<2%). The 5'-triphosphate of CBV was a potent, selective inhibitor of HIV-1 RT, with Ki values for DNA polymerases (alpha, beta, gamma, and epsilon which were 90-, 2,900-, 1,200-, and 1,900-fold greater, respectively, than for RT (Ki, 21 nM). 1592U89 was relatively nontoxic to human bone marrow progenitors erythroid burst-forming unit and granulocyte-macrophage CFU (IC50s, 110 microM) and human leukemic and liver tumor cell lines[1]. Clinical trial: Estimate The Effect Of Lersivirine On The Pharmacokinetics Of Abacavir + Lamivudine In Healthy Subjects. Phage1
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- Duloxetine HCl
Catalog No.:BCC3773
CAS No.:136434-34-9
- SR 27897
Catalog No.:BCC7277
CAS No.:136381-85-6
- Isophysalin A
Catalog No.:BCN7916
CAS No.:1363398-67-7
- Tiotropium Bromide
Catalog No.:BCC2000
CAS No.:136310-93-5
- Methyl 3-amino-2-[[(2'-cyanobiphenyl-4-yl)methyl]amino]benzoate
Catalog No.:BCC9037
CAS No.:136304-78-4
- Ethyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC8965
CAS No.:136285-67-1
- Ethyl2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate
Catalog No.:BCC8978
CAS No.:136285-65-9
- Boc-ß-HoArg(Tos)-OH
Catalog No.:BCC3227
CAS No.:136271-81-3
- TAT 14
Catalog No.:BCC6295
CAS No.:1362661-34-4
- GNE-617
Catalog No.:BCC4280
CAS No.:1362154-70-8
- 11β-Hydroxy-2'-methyl-5'βH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione
Catalog No.:BCC8435
CAS No.:13649-88-2
- Rink Amide Resin
Catalog No.:BCC2570
CAS No.:13653-84-4
- BQ-123
Catalog No.:BCC6963
CAS No.:136553-81-6
- Fmoc-D-Trp-OPfp
Catalog No.:BCC3560
CAS No.:136554-94-4
- Anemarsaponin E
Catalog No.:BCN6290
CAS No.:136565-73-6
- Curdione
Catalog No.:BCN5936
CAS No.:13657-68-6
- Irinotecan HCl Trihydrate
Catalog No.:BCC5091
CAS No.:136572-09-3
- Spermine NONOate
Catalog No.:BCC6950
CAS No.:136587-13-8
- Fmoc-Tyr(3-NO2)-OH
Catalog No.:BCC3280
CAS No.:136590-09-5
- Senexin A
Catalog No.:BCC7980
CAS No.:1366002-50-7
- Calpain Inhibitor II, ALLM
Catalog No.:BCC1234
CAS No.:136632-32-1
- UK 78282 hydrochloride
Catalog No.:BCC7784
CAS No.:136647-02-4
Enantioselective and Regiodivergent Addition of Purines to Terminal Allenes: Synthesis of Abacavir.[Pubmed:28079946]
Angew Chem Int Ed Engl. 2017 Feb 1;56(6):1520-1524.
The rhodium-catalyzed atom-economic asymmetric N-selective intermolecular addition of purine derivatives to terminal allenes is reported. Branched allylic purines were obtained in high yields, regioselectivity and outstanding enantioselectivity utilizing a Rh/Josiphos catalyst. Conversely, linear selective allylation of purines could be realized in good to excellent regio- and E/Z-selectivity with a Pd/dppf catalyst system. Furthermore, the new methodology was applied to a straightforward asymmetric synthesis of carbocyclic nucleoside Abacavir.
[Biliary and kidney lithiasis during treatment with daclatasvir/sofosbuvir/ribavirin and atazanavir/ritonavir + abacavir/lamivudine in an HIV/HCV genotype 4-infected patient: a case report.][Pubmed:28287204]
Recenti Prog Med. 2017 Feb;108(2):98-100.
New Direct-acting Antiviral Agents (DAA)-based anti-HCV therapies currently provide extraordinary opportunities to cure patients. Drug-drug interactions are however a real challenge during treatment. In particular, in HIV-infected patients in cART, DAA choice is limited by such interactions, which can result both in reduced efficacy and toxicity. We report the case of a HIV-infected patient on cART with atazanavir/ritonavir/Abacavir/lamivudine, who presented kidney and biliary lithiasis, the latter treated with endoscopic retrograde cholangiopancreatography and endoscopic biliary sphincterotomy, after beginning anti-HCV treatment with daclatasvir/sofosbuvir/ribavirin. Hyperbilirubinemia with or without jaundice is a well known side effect of atazanavir, because of its inhibition of uridine diphosphate-glucuronosyl transferase. We speculate that in this case hyperbilirubinemia worsening was due to atazanavir/ribavirin co-administration. However, pharmacokinetic data are lacking about atazanavir/daclatasvir concomitant administration in real life setting.
Correction: Abacavir/Lamivudine plus Rilpivirine Is an Effective and Safe Strategy for HIV-1 Suppressed Patients: 48 Week Results of the SIMRIKI Retrospective Study.[Pubmed:28182783]
PLoS One. 2017 Feb 9;12(2):e0172184.
[This corrects the article DOI: 10.1371/journal.pone.0164455.].
Abacavir induces platelet-endothelium interactions by interfering with purinergic signalling: A step from inflammation to thrombosis.[Pubmed:28263802]
Antiviral Res. 2017 May;141:179-185.
The controversy connecting Abacavir (ABC) with cardiovascular disease has been fuelled by the lack of a credible mechanism of action. ABC shares structural similarities with endogenous purines, signalling molecules capable of triggering prothrombotic/proinflammatory programmes. Platelets are leading actors in the process of thrombosis. Our study addresses the effects of ABC on interactions between platelets and other vascular cells, while exploring the adhesion molecules implicated and the potential interference with the purinergic signalling pathway. The effects of ABC on platelet aggregation and platelet-endothelium interactions were evaluated, respectively, with an aggregometer and a flow chamber system that reproduced conditions in vivo. The role of adhesion molecules and purinergic receptors in endothelial and platelet populations was assessed by selective pre-incubation with specific antagonists and antibodies. ABC and carbovir triphosphate (CBT) levels were evaluated by HPLC. The results showed that ABC promoted the adherence of platelets to endothelial cells, a crucial step for the formation of thrombi. This was not a consequence of a direct effect of ABC on platelets, but resulted from activation of the endothelium via purinergic ATP-P2X7 receptors, which subsequently triggered an interplay between P-selectin and ICAM-1 on endothelial cells with constitutively expressed GPIIb/IIIa and GPIbalpha on platelets. ABC did not induce platelet activation (P-selectin expression or Ca(2+) mobilization) or aggregation, even at high concentrations. CBT levels in endothelial cells were lower than those required to induce platelet-endothelium interactions. Thus, ABC interference with endothelial purinergic signalling leads to platelet recruitment. This highlights the endothelium as the main cell target of ABC in this interaction, which is in line with previous experimental evidence that ABC induces manifestations of vascular inflammation.


