Amyloid Beta-peptide (25-35) (human)Functional domain of Aβ CAS# 131602-53-4 |
- Amyloid β-Peptide (10-20) (human)
Catalog No.:BCC1026
CAS No.:152286-31-2
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 131602-53-4 | SDF | Download SDF |
| PubChem ID | 10843733 | Appearance | Powder |
| Formula | C45H81N13O14S | M.Wt | 1060.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | beta-amyloid 25-35 peptide | ||
| Solubility | DMSO : 100 mg/mL (94.32 mM; Need ultrasonic) H2O : 3.33 mg/mL (3.14 mM; Need ultrasonic) | ||
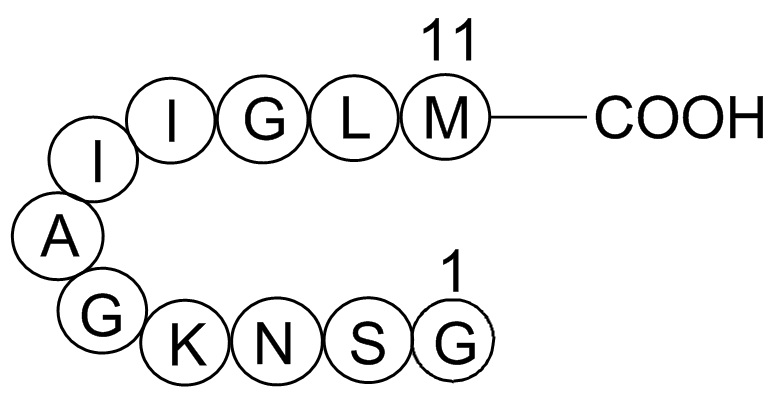
| Sequence | GSNKGAIIGLM | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[2-[[(2S,3S)-2-[[(2S,3S)-2-[[(2S)-2-[[2-[[(2S)-6-amino-2-[[(2S)-4-amino-2-[[(2S)-2-[(2-aminoacetyl)amino]-3-hydroxypropanoyl]amino]-4-oxobutanoyl]amino]hexanoyl]amino]acetyl]amino]propanoyl]amino]-3-methylpentanoyl]amino]-3-methylpentanoyl]amino]acetyl]amino]-4-methylpentanoyl]amino]-4-methylsulfanylbutanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(C(C)CC)C(=O)NCC(=O)NC(CC(C)C)C(=O)NC(CCSC)C(=O)O)NC(=O)C(C)NC(=O)CNC(=O)C(CCCCN)NC(=O)C(CC(=O)N)NC(=O)C(CO)NC(=O)CN | ||
| Standard InChIKey | WIHBNMPFWRHGDF-SLVFWPMISA-N | ||
| Standard InChI | InChI=1S/C45H81N13O14S/c1-9-24(5)36(43(69)50-21-35(63)52-29(17-23(3)4)40(66)55-28(45(71)72)14-16-73-8)58-44(70)37(25(6)10-2)57-38(64)26(7)51-34(62)20-49-39(65)27(13-11-12-15-46)54-41(67)30(18-32(48)60)56-42(68)31(22-59)53-33(61)19-47/h23-31,36-37,59H,9-22,46-47H2,1-8H3,(H2,48,60)(H,49,65)(H,50,69)(H,51,62)(H,52,63)(H,53,61)(H,54,67)(H,55,66)(H,56,68)(H,57,64)(H,58,70)(H,71,72)/t24-,25-,26-,27-,28-,29-,30-,31-,36-,37-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fragment of human amyloid β-peptide, functionally required for the neurotrophic and neurotoxic effects associated with Alzheimer's disease. |

Amyloid Beta-peptide (25-35) (human) Dilution Calculator

Amyloid Beta-peptide (25-35) (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Met-Leu-Gly-Ile-Ile-Ala-Gly-Lys-Asn-Ser-Gly
Amyloid- β (Aβ) peptide is commonly found in human Alzheimer’s disease (AD) brain and is the main component of Alzheimer amyloid plaques. The predominant forms of Aβ in the human brain are Aβ (1-40) and Aβ (1-42). However, the Aβ (25-35) fragment, which is physiologically present in elderly people, is the more toxic region and has recently been found to play a relevant role in AD due to its peculiar aggregation properties1.
Aβ (25-35) is regarded to be the functional domain of Aβ, responsible for its neurotoxic properties2-5. It represents the actual biologically active region of Aβ6. Administration of Aβ (25-35) has been shown to lead to amnesia in mice, causing impairments of spatial working memory along with the degradation of passive avoidance reactions2-5.
In vivo, Aβ (25-35) is present in neurons of subiculum and entorhinal cortex of AD brains7. It is also observed in Inclusion-Body Myositis (IBM) muscle8.
Figure1. Structure of Amyloid β-Peptide
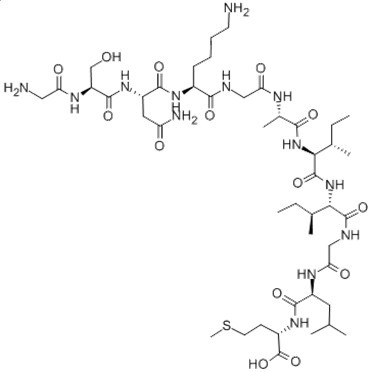
Figure2. Formula of Amyloid β-Peptide (25-35) (human)
C45H81N13O14S
Ref:
1. Millucci L, Ghezzi L, Bernardini G, Santucci A (2010) Conformations and biological activities of amyloid beta peptide 25–35. Curr Protein Pept Sc 11: 54–67.
2. Stepanichev, M.Y.; Moiseeva, Y.V.; Lazareva, N.A.; Gulyaeva,N.V. Studies of the effects of fragment (25-35) of beta-amyloid peptide on the behavior of rats in a radial maze. Neurosci. Behav. Physiol., 2005, 35(5), 511-8.
3. Limón, I.D.; Díaz, A.; Mendieta. L.; Chamorro, G.; Espinosa, B.; Zenteno, E.; Guevara, J. Amyloid-beta(25-35) impairs memory and increases NO in the temporal cortex of rats. Neurosci. Res., 2009,63(2), 129-137.
4. Pike, C. J.; Burdick, D.; Walencewicz, A. J.; Glabe, C. G.; Cotman, C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J. Neurosci., 1993, 13, 1676-1687.
5. Stepanichev, M.Yu; Lazareva N.A.; Onufriev, M.V.; Mitrokhina, O.S.; Moiseeva, Yu.V.; Gulyaeva, N.V. Effects of doses of fragment (25-35) of beta-amyloid peptide on behavior in rats. Neurosci. Behav. Physiol., 1998, 28(5), 564-6 .
6. D'Errico, G.; Vitiello, G.; Ortona, O.; Tedeschi, A.; Ramunno, A. and D'Ursi, A.M. Interaction between Alzheimer's A(25-35) peptide and phospholipid bilayers: The role of cholesterol. Biochimica. Biophys. Acta (BBA) – Biomembr., 2008, 1778, 2710-2716.
7. Kaneko, I.; Yamada, N.; Usui, Y.; Oda, T. Possible involvement of β -amyloids racemized at Ser residue in Alzheimer’s disease. Alzheimer’s Disease: Biology, Diagnose and Therapeutics. John Wiley & Sons: Chichester, 1997, pp. 519-528.
8. Kaneko, I.; Kubo, T.; Morimoto, K.; Kumagae, Y.; Miller, C.A. Ananimal model for Alzheimer’s disease using racemic
- Betulinaldehyde
Catalog No.:BCN1249
CAS No.:13159-28-9
- Calystegine A3
Catalog No.:BCN1884
CAS No.:131580-36-4
- Camelliaside B
Catalog No.:BCN3872
CAS No.:131573-90-5
- Triumbelletin
Catalog No.:BCN6779
CAS No.:131559-54-1
- VU 591 hydrochloride
Catalog No.:BCC6126
CAS No.:1315380-70-1
- NPEC-caged-(1S,3R)-ACPD
Catalog No.:BCC7653
CAS No.:1315379-60-2
- Rac1 Inhibitor F56, control peptide
Catalog No.:BCC5887
CAS No.:1315378-77-8
- pep2-SVKE
Catalog No.:BCC5785
CAS No.:1315378-76-7
- MNI caged kainic acid
Catalog No.:BCC7297
CAS No.:1315378-75-6
- Bax inhibitor peptide, negative control
Catalog No.:BCC2395
CAS No.:1315378-74-5
- PDZ1 Domain inhibitor peptide
Catalog No.:BCC5883
CAS No.:1315378-73-4
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- (+)-Pteryxin
Catalog No.:BCN3470
CAS No.:13161-75-6
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- ACY-241
Catalog No.:BCC6460
CAS No.:1316215-12-9
- OPC 21268
Catalog No.:BCC7812
CAS No.:131631-89-5
- Chalepensin
Catalog No.:BCN7334
CAS No.:13164-03-9
- Turmeronol A
Catalog No.:BCN6777
CAS No.:131651-37-1
- 8-(1-Chloro-2-hydroxy-3-methylbut-3-enyl)-7-methoxycoumarin
Catalog No.:BCN7058
CAS No.:131652-35-2
- Parsonsianine
Catalog No.:BCN2110
CAS No.:131683-36-8
- (R)-(-)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8392
CAS No.:131685-53-5
- Arbidol HCl
Catalog No.:BCC3722
CAS No.:131707-23-8
- Urolignoside
Catalog No.:BCN6758
CAS No.:131723-83-6
- Flavopiridol hydrochloride
Catalog No.:BCC3925
CAS No.:131740-09-5
Inhibitory Effect of Lychee Seed Saponins on Apoptosis Induced by Abeta25-35 through Regulation of the Apoptotic and NF-kappaB Pathways in PC12 Cells.[Pubmed:28353652]
Nutrients. 2017 Mar 29;9(4). pii: nu9040337.
Neuronal apoptosis plays a critical role in the pathogenesis of Alzheimer's disease (AD). Previous studies have shown that lychee seed saponins (LSS), isolated and extracted from traditional Chinese medicine lychee seeds, possess many beneficial activities including anti-oxidation, anti-diabetes, anti-AD, etc. In the present study, we established an in vitro neuronal apoptotic model of PC12 cells induced by Abeta25-35 and studied the effect of LSS on apoptosis by the methods of Hoechst 33342/propidium iodide (PI) fluorescence double staining, Annexin V/PI double staining, and terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL). We also investigated the effects of LSS on mitochondria membrane potential, the expressions of Bcl-2 and Bax proteins, and the mRNA expression and the nuclear translocation of NF-kappaBp65 in PC12 cells. The results showed that LSS markedly inhibited apoptosis, improved the mitochondria membrane potentials, upregulated the expression of Bcl-2 protein, downregulated the expression of Bax protein, and decreased the mRNA expression and nuclear translocation of NF-kappaBp65 in PC12 cells. The study demonstrated that LSS significantly inhibited apoptosis induced by Abeta25-35 via regulation of the apoptotic and NF-kappaB pathways in PC12 cells. Therefore, LSS has the potential to be developed as a novel agent or nutrient supplement for the prevention and/or treatment of AD.
Ciproxifan improves cholinergic transmission, attenuates neuroinflammation and oxidative stress but does not reduce amyloid level in transgenic mice.[Pubmed:28501482]
Life Sci. 2017 Jul 1;180:23-35.
AIM: The present study is aimed to investigate the ability of ciproxifan, a histamine H3 receptor antagonist to inhibit beta-amyloid (Abeta)-induced neurotoxicity in SK-N-SH cells and APP transgenic mouse model. MATERIALS AND METHODS: In vitro studies was designed to evaluate the neuroprotective effects of ciproxifan in Abeta25-35 - induced SK-N-SH cells. For the in vivo study, ciproxifan (1 and 3mg/kg, i.p.) was administrated to transgenic mice for 15days and behaviour was assessed using the radial arm maze (RAM). Brain tissues were collected to measure Abeta levels (Abeta1-40 and Abeta1-42), acetylcholine (ACh), acetylcholinesterase (AChE), nitric oxide (NO), lipid peroxidation (LPO), antioxidant activities, cyclooxygenases (COX) and cytokines (IL-1alpha, IL-1beta and IL-6), while plasma was collected to measure TGF-1beta. RESULTS: The in vitro studies demonstrated neuroprotective effect of ciproxifan by increasing cell viability and inhibiting reactive oxygen species (ROS) in Abeta25-35-induced SK-N-SH cells. Ciproxifan significantly improved the behavioural parameters in RAM. Ciproxifan however, did not alter the Abeta levels in APP transgenic mice. Ciproxifan increased ACh and showed anti-oxidant properties by reducing NO and LPO levels as well as enhancing antioxidant levels. The neuroinflammatory analysis showed that ciproxifan reduced both COX-1 and COX-2 activities, decreased the level of pro-inflammatory cytokines IL-1alpha, IL-1beta and IL-6 and increased the level of anti-inflammatory cytokine TGF-1beta. CONCLUSION: This present study provides scientific evidence of the use of ciproxifan via antioxidant and cholinergic pathways in the management of AD.
The PTEN inhibitor bpV(pic) promotes neuroprotection against amyloid beta-peptide (25-35)-induced oxidative stress and neurotoxicity.[Pubmed:28436304]
Neurol Res. 2017 Aug;39(8):758-765.
OBJECTIVES: The aim of this study was to elucidate the mechanism underlying the neuroprotective effects of the phosphatase and tensin homolog (PTEN) inhibitor, bisperoxovanadium-pic [bpV(pic)]. METHODS: We determined the effects of bpV(pic) on amyloid-beta-peptide-(25-35)-induced neurotoxicity, particularly intracellular reactive oxygen species (ROS) production and mitochondria-mediated apoptotic signaling, in a human neuroblastoma (SH-SY5Y) cell model. RESULTS: We found that exposure of SH-SY5Y cells to amyloid beta peptides (Abeta25-35) resulted in a significant reduction in cell viability accompanied by increased lactate dehydrogenase (LDH) release, elevated levels of intracellular ROS, and decreased superoxide dismutase (SOD) activities, all of which were reversed by co-treatment with bpV(pic). Moreover, bpV(pic) induced significant protection against Abeta25-35-induced apoptosis, and effectively suppressed mitochondria-dependent apoptotic signaling triggered by Abeta25-35. DISCUSSION: Abeta peptides are thought to cause neurodegeneration in Alzheimer's disease (AD), via the induction of free radical oxidative stress. Our results indicate that bpV(pic) provides protection against Abeta25-35-induced oxidative stress and neurotoxicity, suggesting that bpV(pic) could be a potential therapeutic candidate in the treatment of neurodegenerative diseases such as AD.
Curcumin revitalizes Amyloid beta (25-35)-induced and organophosphate pesticides pestered neurotoxicity in SH-SY5Y and IMR-32 cells via activation of APE1 and Nrf2.[Pubmed:28861684]
Metab Brain Dis. 2017 Dec;32(6):2045-2061.
Amyloid beta (Abeta) peptide deposition is the primary cause of neurodegeneration in Alzheimer's disease (AD) pathogenesis. Several reports point towards the role of pesticides in the AD pathogenesis, especially organophosphate pesticides (OPPs). Monocrotophos (MCP) and Chlorpyrifos (CP) are the most widely used OPPs. In this study, the role of MCP and CP in augmenting the Abeta-induced oxidative stress associated with the neurodegeneration in AD has been assessed in human neuroblastoma IMR-32 and SH-SY5Y cell lines. From the cell survival assay, it was observed that MCP and CP reduced cell survival both dose- and time-dependently. Nitro blue tetrazolium (NBT) based assay for determination of intracellular reactive oxygen species (ROS) demonstrated that Abeta(25-35), MCP or CP produce significant oxidative stress alone or synergistically in IMR-32 and SH-SY5Y cells, while pretreatment of curcumin reduced ROS levels significantly in all treatment combinations. In this study, we also demonstrate that treatment of Abeta(25-35) and MCP upregulated inducible nitric oxide synthase (iNOS/NOS2) whereas, no change was observed in neuronal nitric oxide synthase (nNOS/NOS1), but down-regulation of the nuclear factor erythroid 2-related factor 2 (Nrf2) level was observed. While curcumin pretreatment resulted in upregulation of iNOS and Nrf2 proteins. Also, the expression of key DNA repair enzymes APE1, DNA polymerase beta (Pol beta), and PARP1 were found to be downregulated upon treatment with MCP, Abeta(25-35) and their combinations at 24 h and 48 h time points. In this study, pretreatment of curcumin to the SH-SY5Y cells enhanced the expression of DNA repair enzymes APE1, pol beta, and PARP1 enzymes to counter the oxidative DNA base damage via base excision repair (BER) pathway, and also activated the antioxidant element (ARE) via Nrf2 upregulation. Furthermore, the immunofluorescent confocal imaging studies in SH-SY5Y and IMR-32 cells treated with Abeta(25-35) and MCP-mediated oxidative stress and their combinations at different time periods suggesting for cross-talk between the two proteins APE1 and Nrf2. The APE1's association with Nrf2 might be associated with the redox function of APE1 that might be directly regulating the ARE-mediated neuronal survival mechanisms.
Intracerebral beta-amyloid(25-35) produces tissue damage: is it neurotoxic?[Pubmed:1281289]
Neurobiol Aging. 1992 Sep-Oct;13(5):591-4.
beta-Amyloid (1-40) and (25-35) have been reported to be toxic to primary cultured neurons. beta-Amyloid (1-40) was also reported to induce neurodegeneration following intracerebral injection. We attempted to replicate and extend these findings by injecting both the full length amyloid peptide and the 25-35 fragment. beta 1-40 (3 nmol in 1 microliter) or beta 25-35 (20 nmol in 2 microliters) in a vehicle of 10% DMSO (3 and 10 mM concentration, respectively) induced tissue loss and neurodegeneration. We also attempted to prevent the amyloid-induced damage by coinjecting 200 nmol of Substance P. There was no obvious reduction in the size of the lesions. Other studies, however, have reported antagonism of amyloid toxicity with tachykinin agonists. Since beta-amyloid does not appear to bind to tachykinin receptors, there is some question as to the site of the putative interaction of these peptides and, therefore, the mechanism by which beta-amyloid induces tissue damage. Our own results and published cell culture toxicity studies suggest that aggregation of the peptide and physical displacement of tissue may be responsible for both the neuronal and tissue loss, although this hypothesis is not consistent with other published findings.
Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides.[Pubmed:2218531]
Science. 1990 Oct 12;250(4978):279-82.
The amyloid beta protein is deposited in the brains of patients with Alzheimer's disease but its pathogenic role is unknown. In culture, the amyloid beta protein was neurotrophic to undifferentiated hippocampal neurons at low concentrations and neurotoxic to mature neurons at higher concentrations. In differentiated neurons, amyloid beta protein caused dendritic and axonal retraction followed by neuronal death. A portion of the amyloid beta protein (amino acids 25 to 35) mediated both the trophic and toxic effects and was homologous to the tachykinin neuropeptide family. The effects of the amyloid beta protein were mimicked by tachykinin antagonists and completely reversed by specific tachykinin agonists. Thus, the amyloid beta protein could function as a neurotrophic factor for differentiating neurons, but at high concentrations in mature neurons, as in Alzheimer's disease, could cause neuronal degeneration.


