AnastrozoleAromatase inhibitor CAS# 120511-73-1 |
- Exemestane
Catalog No.:BCC1061
CAS No.:107868-30-4
- Letrozole
Catalog No.:BCC1063
CAS No.:112809-51-5
- Aminoglutethimide
Catalog No.:BCC4368
CAS No.:125-84-8
- Formestane
Catalog No.:BCC4369
CAS No.:566-48-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 120511-73-1 | SDF | Download SDF |
| PubChem ID | 2187 | Appearance | Powder |
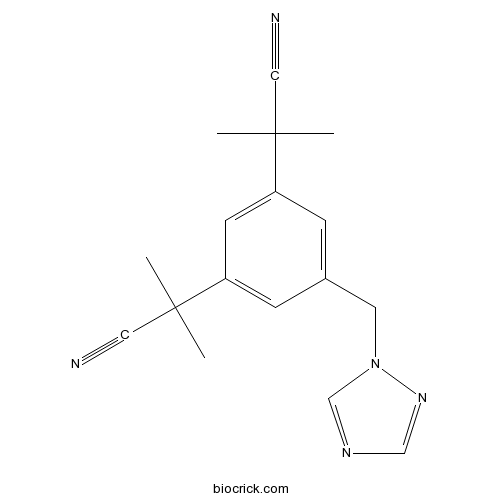
| Formula | C17H19N5 | M.Wt | 293.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ZD1033 | ||
| Solubility | DMSO : ≥ 100 mg/mL (340.87 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-[3-(2-cyanopropan-2-yl)-5-(1,2,4-triazol-1-ylmethyl)phenyl]-2-methylpropanenitrile | ||
| SMILES | CC(C)(C#N)C1=CC(=CC(=C1)CN2C=NC=N2)C(C)(C)C#N | ||
| Standard InChIKey | YBBLVLTVTVSKRW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and highly selective aromatase (CYP19) inhibitor (IC50 = 15 nM) that has no discernible effect on adrenocorticoid hormone synthesis. Reduces plasma estrogen levels and exhibits antitumor activity in vivo. Orally active. |

Anastrozole Dilution Calculator

Anastrozole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4087 mL | 17.0433 mL | 34.0866 mL | 68.1733 mL | 85.2166 mL |
| 5 mM | 0.6817 mL | 3.4087 mL | 6.8173 mL | 13.6347 mL | 17.0433 mL |
| 10 mM | 0.3409 mL | 1.7043 mL | 3.4087 mL | 6.8173 mL | 8.5217 mL |
| 50 mM | 0.0682 mL | 0.3409 mL | 0.6817 mL | 1.3635 mL | 1.7043 mL |
| 100 mM | 0.0341 mL | 0.1704 mL | 0.3409 mL | 0.6817 mL | 0.8522 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Anastrozole (Arimidex,ZD1033) is a potent and selective inhibitor of aromatase with an IC50 value of 14.6 nM or 0.0043μg/ml [1].
Anastrozole has been reported to inhibit human placental aromatase with an IC50 value of 14.6 nM or 0.0043μg/ml. In addition, an oral concentration of 0.1mg/kg of anastrozole has been revealed to completely inhibit ovulation by given on day 2 or day 3 of the cycle. In immature rat, an oral concentration of 0.1mg/kg of anastrozole has also noted to completely extinguish the uterotrophic activity of exogenous AD. Apart from these, by guinea pig, dog and cow adrenal microsomes, anastrozole has been exhibited to suppress the conversion of 11-deoxycortisol to cortisol with mean IC50 values of 4.09μM,129μM and 11.9μM, respectively [1].
References:
[1] Dukes M1, Edwards PN, Large M, Smith IK, Boyle T. The preclinical pharmacology of "Arimidex" (anastrozole; ZD1033)--a potent, selective aromatase inhibitor. J Steroid Biochem Mol Biol. 1996 Jul;58(4):439-45.
- Desacetyldoronine
Catalog No.:BCN2105
CAS No.:120481-77-8
- SRT3190
Catalog No.:BCC1966
CAS No.:1204707-73-2
- SRT3109
Catalog No.:BCC1965
CAS No.:1204707-71-0
- Epacadostat
Catalog No.:BCC6531
CAS No.:1204669-58-8
- Pinanediol talabostat boronate
Catalog No.:BCC1640
CAS No.:1204669-37-3
- Ganoderenic acid H
Catalog No.:BCN2447
CAS No.:120462-48-8
- Ganoderenic acid F
Catalog No.:BCN2446
CAS No.:120462-47-7
- Lophanthoidin F
Catalog No.:BCN6093
CAS No.:120462-46-6
- Lophanthoidin E
Catalog No.:BCN6092
CAS No.:120462-45-5
- Lophanthoidin B
Catalog No.:BCN6091
CAS No.:120462-42-2
- ER 27319 maleate
Catalog No.:BCC5914
CAS No.:1204480-26-1
- (±)-Vesamicol hydrochloride
Catalog No.:BCC6737
CAS No.:120447-62-3
- 16-Nor-7,15-dioxodehydroabietic acid
Catalog No.:BCN7295
CAS No.:120591-53-9
- GLPG0634 analogue
Catalog No.:BCC6547
CAS No.:1206101-20-3
- GLPG0634
Catalog No.:BCC4145
CAS No.:1206161-97-8
- TC Mps1 12
Catalog No.:BCC7974
CAS No.:1206170-62-8
- Mofegiline hydrochloride
Catalog No.:BCC5412
CAS No.:120635-25-8
- 2beta-Acetoxyferruginol
Catalog No.:BCN7955
CAS No.:1206461-56-4
- 4,4'-Dihydroxy-3,3',9-trimethoxy-9,9'-epoxylignan
Catalog No.:BCN7017
CAS No.:1206464-65-4
- N-Benzylnaltrindole hydrochloride
Catalog No.:BCC6782
CAS No.:1206487-81-1
- MK-5172 potassium salt
Catalog No.:BCC1764
CAS No.:1206524-86-8
- Geranyl ferulate
Catalog No.:BCN7078
CAS No.:1206615-69-1
- (RS)-PPG
Catalog No.:BCC6975
CAS No.:120667-15-4
- DMH-1
Catalog No.:BCC5329
CAS No.:1206711-16-1
Can we use gonadotropin plasma concentration as surrogate marker for BMI-related incomplete estrogen suppression in breast cancer patients receiving anastrozole?[Pubmed:28351392]
BMC Cancer. 2017 Mar 28;17(1):226.
BACKGROUND: BMI has been suggested to impact on estrogenic activity in patients receiving Anastrozole resulting in a reduced treatment efficacy in obese women. Current evidence in this regard is controversially discussed. Since estradiol is inversely correlated with gonadotropins it can be assumed that an impact of BMI is also reflected by gonadotropin plasma concentrations. We aim at investigating the impact of BMI on the hormonal state of breast cancer (BC) patients receiving Anastrozole indicated by LH, FSH and SHBG as well as estradiol. METHODS: We determined gonadotropin-, estradiol- and Anastrozole- serum concentrations from postmenopausal, early stage breast cancer patients receiving upfront Anastrozole within routine after care. Gonadotropin plasma concentrations were derived from the routine laboratory examination report. A liquid chromatography tandem mass spectrometry method was used for the measurement of Anastrozole serum concentrations. BMI was assessed within the routine after-care check-up. RESULTS: The overall sample comprised 135 BC patients with a mean age of 65.3 years. BMI was significantly correlated with LH, FSH and SHBG. This association was neither influenced by age nor by Anastrozole serum concentrations according to the regression model. Despite aromatase inhibition 12% of patients had detectable estrogen levels in routine quantification. CONCLUSION: Obese women have an altered hormonal situation compared to normally weight women under the same dose of Anastrozole. Our study findings are a further indicator for the relevance of BMI in regard of Anastrozole metabolism and possible estrogenic activity indicated by gonadotropin plasma level.
NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer.[Pubmed:28270497]
Clin Cancer Res. 2017 Aug 1;23(15):4055-4065.
Purpose: Cyclin-dependent kinase (CDK) 4/6 drives cell proliferation in estrogen receptor-positive (ER(+)) breast cancer. This single-arm phase II neoadjuvant trial (NeoPalAna) assessed the antiproliferative activity of the CDK4/6 inhibitor palbociclib in primary breast cancer as a prelude to adjuvant studies.Experimental Design: Eligible patients with clinical stage II/III ER(+)/HER2(-) breast cancer received Anastrozole 1 mg daily for 4 weeks (cycle 0; with goserelin if premenopausal), followed by adding palbociclib (125 mg daily on days 1-21) on cycle 1 day 1 (C1D1) for four 28-day cycles unless C1D15 Ki67 > 10%, in which case patients went off study due to inadequate response. Anastrozole was continued until surgery, which occurred 3 to 5 weeks after palbociclib exposure. Later patients received additional 10 to 12 days of palbociclib (Cycle 5) immediately before surgery. Serial biopsies at baseline, C1D1, C1D15, and surgery were analyzed for Ki67, gene expression, and mutation profiles. The primary endpoint was complete cell cycle arrest (CCCA: central Ki67 Anastrozole (C1D15 87% vs. C1D1 26%, P < 0.001). Palbociclib enhanced cell-cycle control over Anastrozole monotherapy regardless of luminal subtype (A vs. B) and PIK3CA status with activity observed across a broad range of clinicopathologic and mutation profiles. Ki67 recovery at surgery following palbociclib washout was suppressed by cycle 5 palbociclib. Resistance was associated with nonluminal subtypes and persistent E2F-target gene expression.Conclusions: Palbociclib is an active antiproliferative agent for early-stage breast cancer resistant to Anastrozole; however, prolonged administration may be necessary to maintain its effect. Clin Cancer Res; 23(15); 4055-65. (c)2017 AACR.
Ki-67 antigen expression in the mammary epithelium of female rats in persistent estrus treated with anastrozole.[Pubmed:28277134]
Gynecol Endocrinol. 2017 May;33(5):359-362.
OBJECTIVES: To evaluate Ki-67 antigen expression in mammary epithelium of female rats in persistent estrus treated with Anastrozole. MATERIALS AND METHODS: Twenty-eight Wistar-Hanover female rats in persistent estrus induced by subcutaneous injection of 1.25 mg of testosterone propionate in the second day of life were randomly divided into two groups, control and experimental, with 14 animals each. The animals of control group received only the vehicle (propyleneglycol) and the animals of group experimental received 0.125 mg daily of Anastrozole by gavage during 28 days. After 28 days of treatment, all animals were sacrificed and the first pair of abdominal-inguinal mammary glands was removed and fixed in 10% buffered formalin to investigate Ki-67 antigen expression by immunohistochemistry. RESULTS: The mean percentage of Ki-67-stained nuclei per 500 cells in the mammary epithelium was 76.97 +/- 0.76 and 14.44 +/- 2.02 [mean +/- standard error of the mean (SEM)] in the control and experimental groups, respectively (p < 0.0001). CONCLUSIONS: Anastrozole treatment significantly reduced Ki-67 expression in the mammary epithelium of rats in persistent estrus.
Erratum to: A randomized pilot trial of growth hormone with anastrozole versus growth hormone alone, starting at the very end of puberty in adolescents with idiopathic short stature.[Pubmed:28261275]
Int J Pediatr Endocrinol. 2017;2017:4.
[This corrects the article DOI: 10.1186/1687-9856-2015-4.].
Anastrozole (Arimidex)--an aromatase inhibitor for the adjuvant setting?[Pubmed:11900212]
Br J Cancer. 2001 Nov;85 Suppl 2:6-10.
Anastrozole (Arimidex) is a third-generation aromatase inhibitor which has been shown to possess superior efficacy and tolerability over established endocrine agents in advanced breast cancer. Inhibition of aromatase prevents the conversion of androgen substrates to oestrogen, its sole source in postmenopausal women, thereby leading to regression of hormone-sensitive breast carcinomas. Clinical pharmacology data indicate that Anastrozole is a potent aromatase inhibitor, providing near-maximal suppression of serum and intratumoural oestrogens to below detectable levels. Anastrozole may offer greater selectivity compared with other aromatase inhibitors, being without any intrinsic endocrine effects and with no apparent effect on the synthesis of adrenal steroids. It is well tolerated and has a convenient once-daily dosing regimen, ensuring maximum patient compliance. A major clinical programme has demonstrated that Anastrozole is superior to the standard endocrine therapy, tamoxifen, for the first-line treatment of postmenopausal women with hormone-sensitive advanced breast cancer. Its superior efficacy in advanced disease, together with its improved tolerability and convenient dosage, make it a suitable agent to be assessed for the treatment of early breast cancer in postmenopausal women. This was investigated in the largest single adjuvant breast cancer study ever to be carried out, the ATAC (Arimidex, tamoxifen, alone or in combination) trial, which has now completed recruitment, with the first efficacy and safety data awaited.
Anastrozole: a new selective nonsteroidal aromatase inhibitor.[Pubmed:9394367]
Oncology (Williston Park). 1997 Nov;11(11):1697-703; discussion 1707-8.
Aromatase (estrogen synthetase) is the enzyme complex responsible for the final step in estrogen synthesis--the conversion of androstenedione and testosterone to estrone and estradiol, respectively. Inhibitors of this enzyme have been shown to be clinically effective in the treatment of advanced breast cancer in postmenopausal women, in whom the major source of estrogen production derives from aromatization of adrenal androgens in peripheral tissues, such as muscle, liver, and fat. The most widely used aromatase inhibitor has been aminoglutethimide; however, it is nonselective and also inhibits adrenocorticosteroid synthesis, necessitating hydrocortisone supplementation. Aminoglutethimide is also associated with frequent and troublesome side effects. Formestane, the first selective aromatase inhibitor to be developed, has an improved safety profile and selectivity, but its use has been limited somewhat by its inconvenient administration via intramuscular injection. In this article, the preclinical and clinical data published to date on the new third-generation aromatase inhibitor Anastrozole (Arimidex) are presented in the context of current endocrine therapies. Future applications of aromatase inhibitors, both as monotherapy and in combination with other endocrine therapies, are discussed. The use of aromatase inhibitors in advanced disease, the adjuvant setting, and as possible chemopreventive agents are examined.
The preclinical pharmacology of "Arimidex" (anastrozole; ZD1033)--a potent, selective aromatase inhibitor.[Pubmed:8903429]
J Steroid Biochem Mol Biol. 1996 Jul;58(4):439-45.
Anastrozole is a comparatively simple, achiral benzyltriazole derivative, 2,2'-[5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]bis(2-++ +methylpropiononitrile), that inhibits human placental aromatase with an IC50 of 15 nM and elicits maximal activity in vivo in rats (inhibition of ovulation and androstenedione-induced uterine hypertrophy) and monkeys (lowering of plasma oestradiol) at 0.1 mg/kg p.o. At 30 times this dose, Anastrozole does not elevate plasma 11-deoxycorticosterone in monkeys, and at 100 times this dose, does not affect plasma aldosterone levels or Na+/K+ excretion in rats, plasma K+ concentrations in dogs, or cause adrenal hypertrophy in rats or dogs. It therefore has no discernible effect on adrenocorticoid hormone synthesis in vivo at very large multiples of its maximally effective aromatase-inhibiting dose. At similar large multiples in rats it displays no oestrogenic, anti-oestrogenic, androgenic, anti-androgenic, progestogenic, glucocorticoid, antiglucocorticoid or mineralocorticoid activity. Anastrozole is thus a potent and highly selective aromatase inhibitor, with no intrinsic hormonal activities--a pharmacological profile particularly suitable for the treatment of breast cancer.


