BMS-663068HIV-1 attachment inhibitor CAS# 864953-29-7 |
- NBD-556
Catalog No.:BCC1790
CAS No.:333353-44-9
- BMS-378806
Catalog No.:BCC4505
CAS No.:357263-13-9
- BMS-663068 Tris
Catalog No.:BCC1429
CAS No.:864953-39-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 864953-29-7 | SDF | Download SDF |
| PubChem ID | 11319217 | Appearance | Powder |
| Formula | C25H26N7O8P | M.Wt | 583.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Fostemsavir | ||
| Solubility | DMSO : ≥ 100 mg/mL (171.38 mM) H2O : 20 mg/mL (34.28 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
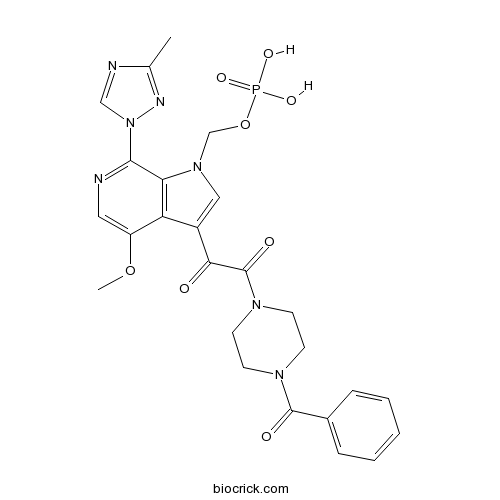
| Chemical Name | [3-[2-(4-benzoylpiperazin-1-yl)-2-oxoacetyl]-4-methoxy-7-(3-methyl-1,2,4-triazol-1-yl)pyrrolo[2,3-c]pyridin-1-yl]methyl dihydrogen phosphate | ||
| SMILES | CC1=NN(C=N1)C2=NC=C(C3=C2N(C=C3C(=O)C(=O)N4CCN(CC4)C(=O)C5=CC=CC=C5)COP(=O)(O)O)OC | ||
| Standard InChIKey | SWMDAPWAQQTBOG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H26N7O8P/c1-16-27-14-32(28-16)23-21-20(19(39-2)12-26-23)18(13-31(21)15-40-41(36,37)38)22(33)25(35)30-10-8-29(9-11-30)24(34)17-6-4-3-5-7-17/h3-7,12-14H,8-11,15H2,1-2H3,(H2,36,37,38) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BMS-663068 is the phosphonooxymethyl prodrug of BMS-626529. BMS-626529 is a novel attachment inhibitor that targets HIV-1 gp120 and prevents its binding to CD4+ T cells.In Vivo:BMS-663068 has good antiviral activity in subjects infected with virus shown to be susceptible (IC50, <100 nM) to the agent[1]. References: | |||||

BMS-663068 Dilution Calculator

BMS-663068 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7138 mL | 8.5691 mL | 17.1383 mL | 34.2765 mL | 42.8456 mL |
| 5 mM | 0.3428 mL | 1.7138 mL | 3.4277 mL | 6.8553 mL | 8.5691 mL |
| 10 mM | 0.1714 mL | 0.8569 mL | 1.7138 mL | 3.4277 mL | 4.2846 mL |
| 50 mM | 0.0343 mL | 0.1714 mL | 0.3428 mL | 0.6855 mL | 0.8569 mL |
| 100 mM | 0.0171 mL | 0.0857 mL | 0.1714 mL | 0.3428 mL | 0.4285 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BMS-663068 is a small-molecule attachment inhibitor of HIV-1 gp120 with IC50 value of [1].
The first step of HIV-1 virus to entry host cells is the binding of the viral gp120 envelope glycopeptide to the CD4 receptor of host cell. BMS-663068 is an attachment inhibitor against specific HIV-1 isolates and targets this step. It is a phosphonooxy methyl prodrug and can be cleaved by alkaline phosphatase in the gut then releases the effective moiety BMS-626529. Compared with the progenitor attachment inhibitorBMS-488043, BMS-663068 has an improved antivirus spectrum [1].
BMS-663068 showed low cytotoxicity in cell culture. In PM1 and PBMC cells, the CC50 values were 105 and 192 μM, respectively. In HEK293, HepG2, HCT116, HeLa, MT-2, MCF-7 and H292 cells, the CC50 values were all higher than 200 μM. It was found that BMS-663068 has potent anti-virus activity against both the laboratory strains and the clinical isolates of HIV. For the CXCR4-tropic LAI virus, BMS-663068 showed EC50 value of 0.7 nM. For a cohort of laboratory strains including NL4-3, SF-162 and JRFL, the inhibition efficacies of BMS-663068 against them were 7 to 10-fold higher than that of BMS-488043. It was even more potent than BMS-488043 when treated with 89.6 viruses (15-fold), Bal virus (14-fold) and MN virus (67-fold). In an antiviral assay using PBMC cells, BMS-663068 was treated against a total of 88 HIV-1 viruses obtained from the NIH AIDS Repository. It exerted an EC50 value of 0.01 nM against the most susceptible virus and an EC50 value of 2μM against the least susceptible virus. In addition, it was found that the virus resistant to other HIV-1 entry inhibitors such as ENF and ibalizumab retained susceptibility to BMS-626529 [1 and 2].
References:
[1] Nowicka-Sans B, Gong YF, McAuliffe B, Dicker I, Ho HT, Zhou N, Eggers B, Lin PF, Ray N, Wind-Rotolo M, Zhu L, Majumdar A, Stock D, Lataillade M, Hanna GJ, Matiskella JD, Ueda Y, Wang T, Kadow JF, Meanwell NA, Krystal M. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother. 2012 Jul;56(7):3498-507.
[2] Li Z, Zhou N, Sun Y, Ray N, Lataillade M, Hanna GJ, Krystal M. Activity of the HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068, against CD4-independent viruses and HIV-1 envelopes resistant to other entry inhibitors. Antimicrob Agents Chemother. 2013 Sep;57(9):4172-80.
- Leojaponin
Catalog No.:BCN7381
CAS No.:864817-63-0
- Resminostat (RAS2410)
Catalog No.:BCC2165
CAS No.:864814-88-0
- Gnetucleistol C
Catalog No.:BCN3395
CAS No.:864763-61-1
- Gnetucleistol B
Catalog No.:BCN3585
CAS No.:864763-60-0
- TC-MCH 7c
Catalog No.:BCC6149
CAS No.:864756-35-4
- SB 699551
Catalog No.:BCC7594
CAS No.:864741-95-7
- Sequosempervirin D
Catalog No.:BCN4562
CAS No.:864719-19-7
- Sequosempervirin B
Catalog No.:BCN4777
CAS No.:864719-17-5
- Nigrolineaxanthone V
Catalog No.:BCN4411
CAS No.:864516-31-4
- Sanggenone H
Catalog No.:BCN2946
CAS No.:86450-80-8
- Sanggenone K
Catalog No.:BCN3373
CAS No.:86450-77-3
- BNTX maleate
Catalog No.:BCC6838
CAS No.:864461-31-4
- BMS-663068 Tris
Catalog No.:BCC1429
CAS No.:864953-39-9
- Vinblastine
Catalog No.:BCN2376
CAS No.:865-21-4
- Gelsempervine A
Catalog No.:BCN3929
CAS No.:865187-17-3
- AMG837
Catalog No.:BCC6387
CAS No.:865231-46-5
- 4,5-dihydroxy-3,8-dimethylnaphthalene-1,2-dione
Catalog No.:BCN8422
CAS No.:86533-36-0
- FR 180204
Catalog No.:BCC3669
CAS No.:865362-74-9
- N-Methylcalycinine
Catalog No.:BCN4412
CAS No.:86537-66-8
- Benazepril HCl
Catalog No.:BCC5019
CAS No.:86541-74-4
- Benazepril
Catalog No.:BCC4286
CAS No.:86541-75-5
- apigenin 7-O-(6〃-O-malonyl)-β-D-glucoside
Catalog No.:BCN8399
CAS No.:86546-87-4
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
- Bisisorhapontigenin A
Catalog No.:BCN3501
CAS No.:865474-98-2
Model-Based Phase 3 Dose Selection for HIV-1 Attachment Inhibitor Prodrug BMS-663068 in HIV-1-Infected Patients: Population Pharmacokinetics/Pharmacodynamics of the Active Moiety, BMS-626529.[Pubmed:26902761]
Antimicrob Agents Chemother. 2016 Apr 22;60(5):2782-9.
BMS-663068 is an oral prodrug of the HIV-1 attachment inhibitor BMS-626529, which prevents viral attachment to host CD4(+) T cells by binding to HIV-1 gp120. To guide dose selection for the phase 3 program, pharmacokinetic/pharmacodynamic modeling was performed using data from two phase 2 studies with HIV-1-infected subjects (n = 244). BMS-626529 population pharmacokinetics were described by a two-compartment model with first-order elimination from the central compartment, zero-order release of prodrug from the extended-release formulation into a hypothetical absorption compartment, and first-order absorption into the central compartment. The covariates of BMS-663068 formulation type, lean body mass, baseline CD8(+) T-cell percentage, and ritonavir coadministration were found to be significant contributors to intersubject variability. Exposure-response analyses showed a relationship between the loge-transformed concentration at the end of a dosing interval (Ctau) normalized for the protein binding-adjusted BMS-626529 half-maximal (50%) inhibitory concentration (PBAIC50) and the change in the HIV-1 RNA level from the baseline level after 7 days of BMS-663068 monotherapy. The probability of achieving a decline in HIV-1 RNA level of >0.5 or >1.0 log10 copies/ml as a function of the loge-transformed PBAIC50-adjusted Ctau after 7 days of monotherapy was 99 to 100% and 57 to 73%, respectively, for proposed BMS-663068 doses of 400 mg twice daily (BID), 600 mg BID (not studied in the phase 2b study), 800 mg BID, 600 mg once daily (QD), and 1,200 mg QD. On the basis of a slight advantage in efficacy of BID dosing over QD dosing, similar responses for the 600- and 800-mg BID doses, and prior clinical observations, BMS-663068 at 600 mg BID was predicted to have the optimal benefit-risk profile and selected for further clinical investigation. (The phase 2a proof-of-concept study AI438006 and the phase 2b study AI438011 are registered at ClinicalTrials.gov under numbers NCT01009814 and NCT01384734, respectively.).
Pharmacokinetic interactions between BMS-626529, the active moiety of the HIV-1 attachment inhibitor prodrug BMS-663068, and ritonavir or ritonavir-boosted atazanavir in healthy subjects.[Pubmed:25870057]
Antimicrob Agents Chemother. 2015 Jul;59(7):3816-22.
BMS-663068 is a prodrug of BMS-626529, a first-in-class attachment inhibitor that binds directly to HIV-1 gp120, preventing initial viral attachment and entry into host CD4(+) T cells. This open-label, multiple-dose, four-sequence, crossover study addressed potential two-way drug-drug interactions following coadministration of BMS-663068 (BMS-626529 is a CYP3A4 substrate), atazanavir (ATV), and ritonavir (RTV) (ATV and RTV are CYP3A4 inhibitors). Thirty-six healthy subjects were randomized 1:1:1:1 to receive one of four treatment sequences with three consecutive treatments: BMS-663068 at 600 mg twice daily (BID), BMS-663068 at 600 mg BID plus RTV at 100 mg once daily (QD), ATV at 300 mg QD plus RTV at 100 mg QD (RTV-boosted ATV [ATV/r]), or BMS-663068 at 600 mg BID plus ATV at 300 mg QD plus RTV at 100 mg QD. Compared with the results obtained by administration of BMS-663068 alone, coadministration of BMS-663068 with ATV/r increased the BMS-626529 maximum concentration in plasma (Cmax) and the area under the concentration-time curve in one dosing interval (AUCtau) by 68% and 54%, respectively. Similarly, coadministration of BMS-663068 with RTV increased the BMS-626529 Cmax and AUCtau by 53% and 45%, respectively. Compared with the results obtained by administration of ATV/r alone, ATV and RTV systemic exposures remained similar following coadministration of BMS-663068 with ATV/r. BMS-663068 was generally well tolerated, and there were no adverse events (AEs) leading to discontinuation, serious AEs, or deaths. Moderate increases in BMS-626529 systemic exposure were observed following coadministration of BMS-663068 with ATV/r or RTV. However, the addition of ATV to BMS-663068 plus RTV did not further increase BMS-626529 systemic exposure. ATV and RTV exposures remained similar following coadministration of BMS-663068 with either ATV/r or RTV. BMS-663068 was generally well tolerated alone or in combination with either RTV or ATV/r.
Safety and efficacy of the HIV-1 attachment inhibitor prodrug BMS-663068 in treatment-experienced individuals: 24 week results of AI438011, a phase 2b, randomised controlled trial.[Pubmed:26423650]
Lancet HIV. 2015 Oct;2(10):e427-37.
BACKGROUND: BMS-663068 is an oral prodrug of BMS-626529, an attachment inhibitor that binds to HIV-1 gp120, blocking viral attachment to host CD4 cells. AI438011 is an ongoing trial investigating the efficacy, safety, and dose-response of BMS-663068 in treatment-experienced, HIV-1-infected patients. Herein we present the results of the primary analysis. METHODS: AI438011 is a phase 2b, randomised, active-controlled trial, at 53 hospitals and outpatient clinics across ten countries in North and South America, Europe, and Africa. Individuals with an HIV-1 RNA viral load of at least 1000 copies per mL and a BMS-626529 half-maximum inhibitory concentration lower than 100 nmol/L were randomly assigned (1:1:1:1:1) to receive either BMS-663068 at 400 mg twice daily, 800 mg twice daily, 600 mg once daily, or 1200 mg once daily or ritonavir-boosted atazanavir (300 mg of atazanavir and 100 mg of ritonavir once daily), each with 400 mg of raltegravir twice daily and 300 mg of tenofovir disoproxil fumarate once daily as a backbone. The sponsor, participants, and investigators were masked for BMS-663068 dose but not for allocation. Primary endpoints were the proportion of patients with an HIV-1 RNA viral load less than 50 copies per mL (response rate) at week 24 and the frequency of serious adverse events and adverse events leading to discontinuation, up to the week 24 analysis. The primary analyses included all patients who received at least one dose of study drug (modified intention-to-treat population). This study is registered at ClinicalTrials.gov, NCT01384734. FINDINGS: Between July 26, 2011, and July 16, 2012, 581 participants were assessed for eligibility. Of these, 254 patients were randomly assigned to receive either BMS-663068 (n=52 for the 400 mg twice daily group, n=50 for the 800 mg twice daily group, n=51 for the 600 mg once daily group, and n=50 for the 1200 mg once daily group) or ritonavir-boosted atazanavir (n=51). 200 patients received at least one dose of BMS-663068, and 51 patients received at least one dose of ritonavir-boosted atazanavir. At week 24, 40 (80%) of 50 patients in the BMS-663068 400 mg twice daily group, 34 (69%) of 49 patients in the 800 mg twice daily group, 39 (76%) of 51 patients in the 600 mg once daily group, and 36 (72%) of 50 patients in the 1200 mg once daily group had an HIV-1 RNA viral load less than 50 copies per mL, compared with 38 (75%) of 51 patients in the ritonavir-boosted atazanavir group. Serious adverse events were noted in 13 (7%) of 200 patients in the BMS-663068 groups and five (10%) of the 51 patients in the ritonavir-boosted atazanavir group. Four (2%) of the 200 patients in the BMS-663068 groups and two (4%) of the 51 patients in the ritonavir-boosted atazanavir group discontinued because of adverse events. No serious adverse events or adverse events leading to discontinuation were BMS-663068-related. Grade 2-4 adverse events related to study drug(s) occurred in 17 (9%) of 200 patients across the BMS-663068 groups and 14 (27%) of 51 patients in the ritonavir-boosted atazanavir group. For the BMS-663068 groups these events were mostly single instances with no dose relation and for the ritonavir-boosted atazanavir group these were mostly gastrointestinal or hepatobiliary disorders associated with hyperbilirubinaemia. INTERPRETATION: In a comparison with ritonavir-boosted atazanavir, efficacy and safety of BMS-663068 up to the week 24 analysis support continued development of BMS-663068, which is being assessed in a phase 3 trial in heavily treatment-experienced individuals. FUNDING: Bristol-Myers Squibb.


