Bafilomycin A1V-ATPase inhibitor,selective and reversible CAS# 88899-55-2 |
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- Concanamycin A
Catalog No.:BCC3919
CAS No.:80890-47-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure
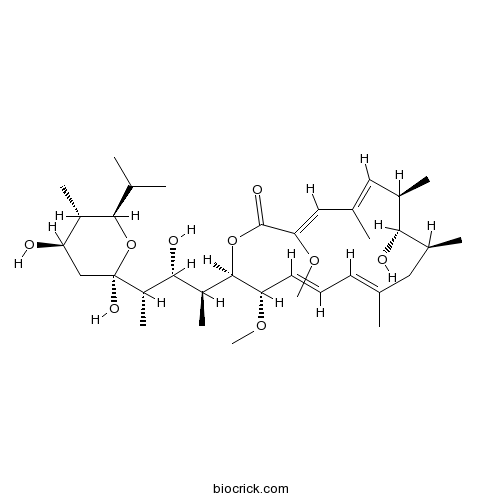
3D structure
| Cas No. | 88899-55-2 | SDF | Download SDF |
| PubChem ID | 6436223 | Appearance | Powder |
| Formula | C35H58O9 | M.Wt | 622.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (-)-Bafilomycin A1 | ||
| Solubility | DMSO : 100 mg/mL (160.56 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (3Z,5E,7R,8S,9S,11E,13E,15S,16R)-16-[(2S,3R,4S)-4-[(2R,4R,5S,6R)-2,4-dihydroxy-5-methyl-6-propan-2-yloxan-2-yl]-3-hydroxypentan-2-yl]-8-hydroxy-3,15-dimethoxy-5,7,9,11-tetramethyl-1-oxacyclohexadeca-3,5,11,13-tetraen-2-one | ||
| SMILES | CC1CC(=CC=CC(C(OC(=O)C(=CC(=CC(C1O)C)C)OC)C(C)C(C(C)C2(CC(C(C(O2)C(C)C)C)O)O)O)OC)C | ||
| Standard InChIKey | XDHNQDDQEHDUTM-JQWOJBOSSA-N | ||
| Standard InChI | InChI=1S/C35H58O9/c1-19(2)32-24(7)27(36)18-35(40,44-32)26(9)31(38)25(8)33-28(41-10)14-12-13-20(3)15-22(5)30(37)23(6)16-21(4)17-29(42-11)34(39)43-33/h12-14,16-17,19,22-28,30-33,36-38,40H,15,18H2,1-11H3/b14-12+,20-13+,21-16+,29-17-/t22-,23+,24-,25-,26-,27+,28-,30-,31+,32+,33+,35+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly potent, selective inhibitor of vacuolar H+-ATPases (IC50 = 0.6 - 1.5 nM in bovine chromaffin granules). Selective for v-ATPase over other ATP hydrolyzing enzymes such as F-ATPases and the H+/K+-ATPase (P-ATPase). Thought to inhibit autophagy either by blocking autophagosome-lysosome fusion (in H4IIE cells), or by blocking lysosomal degradation. |

Bafilomycin A1 Dilution Calculator

Bafilomycin A1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6055 mL | 8.0277 mL | 16.0555 mL | 32.111 mL | 40.1387 mL |
| 5 mM | 0.3211 mL | 1.6055 mL | 3.2111 mL | 6.4222 mL | 8.0277 mL |
| 10 mM | 0.1606 mL | 0.8028 mL | 1.6055 mL | 3.2111 mL | 4.0139 mL |
| 50 mM | 0.0321 mL | 0.1606 mL | 0.3211 mL | 0.6422 mL | 0.8028 mL |
| 100 mM | 0.0161 mL | 0.0803 mL | 0.1606 mL | 0.3211 mL | 0.4014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bafilomycin A1 is a selective inhibitor of vacuolar H+ ATPases (V-ATPases) with I50 values of 4-400 nmol/mg 1.
Bafilomycin A1 was treated to different types of membrane ATPases with the dependent I50 of 400 nmol/mg, 4 nmol/mg and 50 nmol/mg for the vacuolar ATPases of a fungus (N. crassa), a plant (Z. mays), and an animal (bovine abrenal medulla). In addition, the inhibitory effect of bafilomycin A1 on vacuolar ATPases was further determined by testing its influence on H+-pumping activity. H+ translocation was completely inhibited by 10 nM bafilomycin A1 1. Other study has shown that bafilomycin A1 strongly inhibited the pit-forming activity of osteoclasts. The subcellular localization of V-ATPase in osteoclasts was been treated with or without bafilomycin A1 by immunoelectron microscopy. These results suggested that disruption of V-ATPase localization to the ruffled border by bafilomycin A1 resulted in the lack of acidification of the dentine surface 2.
Notes: I50 values is umol of bafilomycin AI per mg of protein giving 50% inhibition of ATPase activity.
References:
1. Bowman E J, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells[J]. Proceedings of the National Academy of Sciences, 1988, 85(21): 7972-7976.
2. Takami M, Suda K, Sahara T, et al. Involvement of vacuolar H+-ATPase in incorporation of risedronate into osteoclasts[J]. Bone, 2003, 32(4): 341-349.
- Atrial natriuretic factor (1-28) (rat)
Catalog No.:BCC5843
CAS No.:88898-17-3
- Inulanolide A
Catalog No.:BCN3705
CAS No.:888941-86-4
- Fosinopril sodium
Catalog No.:BCC2141
CAS No.:88889-14-9
- Sprengerinin A
Catalog No.:BCN6658
CAS No.:88866-99-3
- Adrenorphin, Free Acid
Catalog No.:BCC1011
CAS No.:88866-92-6
- Sprengerinin C
Catalog No.:BCN6657
CAS No.:88861-91-0
- 11,12-De(methylenedioxy)danuphylline
Catalog No.:BCN4436
CAS No.:888482-17-5
- Ganetespib (STA-9090)
Catalog No.:BCC2336
CAS No.:888216-25-9
- Galanin (porcine)
Catalog No.:BCC5960
CAS No.:88813-36-9
- Gambogoic acid B
Catalog No.:BCN7936
CAS No.:887923-50-4
- Isogambogenic acid
Catalog No.:BCN3076
CAS No.:887923-47-9
- Gaudichaudic acid
Catalog No.:BCN3071
CAS No.:887923-46-8
- Mogroside V
Catalog No.:BCN1036
CAS No.:88901-36-4
- Mogroside IIIe
Catalog No.:BCN7924
CAS No.:88901-37-5
- Mogroside IIe
Catalog No.:BCN3168
CAS No.:88901-38-6
- Mogroside IVa
Catalog No.:BCN3165
CAS No.:88901-41-1
- Mogroside III-A1
Catalog No.:BCN3170
CAS No.:88901-42-2
- Mogroside III-A2
Catalog No.:BCN7925
CAS No.:88901-43-3
- Mogroside II-A1
Catalog No.:BCN7926
CAS No.:88901-44-4
- Mogroside II-A2
Catalog No.:BCN3180
CAS No.:88901-45-5
- (-)-Xestospongin C
Catalog No.:BCC7002
CAS No.:88903-69-9
- Mogroside IVe
Catalog No.:BCN3166
CAS No.:88915-64-4
- Mogrol
Catalog No.:BCN8446
CAS No.:88930-15-8
- Dipsanoside A
Catalog No.:BCN2877
CAS No.:889678-62-0
Bafilomycin A1 triggers proliferative potential of senescent cancer cells in vitro and in NOD/SCID mice.[Pubmed:28030837]
Oncotarget. 2017 Feb 7;8(6):9303-9322.
Anticancer therapies that induce DNA damage tend to trigger senescence in cancer cells, a process known as therapy-induced senescence (TIS). Such cells may undergo atypical divisions, thus contributing to tumor re-growth. Accumulation of senescent cancer cells reduces survival of patients after chemotherapy. As senescence interplays with autophagy, a dynamic recycling process, we sought to study whether inhibition of autophagy interferes with divisions of TIS cells. We exposed human colon cancer HCT116 cells to repeated cycles of a chemotherapeutic agent - doxorubicin (doxo) and demonstrated induction of hallmarks of TIS (e.g. growth arrest, hypertrophy, poliploidization and secretory phenotype) and certain properties of cancer stem cells (increased NANOG expression, percentages of CD24+ cells and side population). Colonies of small and highly proliferative progeny appeared shortly after drug removal. Treatment with Bafilomycin A1 (BAF A1), an autophagy inhibitor, postponed short term in vitro cell re-population. It was associated with reduction in the number of diploid and increase in the number of poliploid cells. In a long term, a pulse of BAF A1 resulted in reactivation of autophagy in a subpopulation of HCT116 cells and increased proliferation. Accordingly, the senescent HCT116 cells treated with BAF A1 when injected into NOD/SCID mice formed tumors, in contrast to the controls.Our results suggest that senescent cancer cells that appear during therapy, can be considered as dormant cells that contribute to cancer re-growth, when chemotherapeutic treatment is stopped. These data unveil new mechanisms of TIS-related cancer maintenance and re-population, triggered by a single pulse of BAF A1 treatment.
Dehydropachymic acid decreases bafilomycin A1 induced beta-Amyloid accumulation in PC12 cells.[Pubmed:28077330]
J Ethnopharmacol. 2017 Feb 23;198:167-173.
ETHNOPHARMACOLOGICAL RELEVANCE: Fuling, the sclerotium of Poria cocos, was frequently used in traditional Chinese medicine (TCM) formulae for Alzheimer's disease (AD) intervention over the past 10 centuries. And its extracts exhibited significant effects in both cellular and animal models of AD in previous studies. However, its mechanisms on prevention and treatment of AD have not been well elucidated yet. AIM OF THE STUDY: To investigate the effect and corresponding mechanisms of dehydropachymic acid, which is one of the major triterpenes in P. cocos, on the clearance of beta-amyloid accumulation in Bafilomycin A1 induced PC12 cells. MATERIALS AND METHODS: MTT assay was used to examine the DPA effect on the viability of PC12 cells stable transfected with pCB6-APP (PC12-APP). PC12-APP cells were treated with DPA at the concentration of 6.25, 12.5, 25mug/mL for 4h, and then co-treated with 50nmol/L Bafilomycin A1 for 48h except the controls. The Abeta1-42 content in culture medium was determined by ELISA. The intracellular amount of APP, Abeta1-42, LC3, cathepsin D was measured by Western blotting and normalized to GAPDH loading control. The PC12 cells stable transfected with pSelect-LC3-GFP (PC12-LC3-GFP) was used in the fluorescence microscopy estimation of autophagosomes accumulation. The internal pH in lysosome was detected by LysoTracker Red staining. RESULTS: DPA had no significant effect on the cell viability but could significantly decrease Abeta1-42 content in culture medium and eliminate the intracellular accumulation of APP and Abeta1-42 in Bafilomycin A1 induced PC12-APP cells. Furthermore, DPA lowered the LC3-II/LC3-I ratio and reduced the GFP-labeled LC3 puncta which were elevated by Bafilomycin A1. And the increase in internal pH of lysosome and decrease in mCatD amount in Bafilomycin A1 induced PC12-APP cells were restored by DPA treatment. These results indicated that DPA could restore the lysosomal acidification and recover the autophgic flux which is impaired by Bafilomycin A1. CONCLUSIONS: DPA could effectively clear the accumulation of Abeta1-42 in Bafilomycin A1 impaired PC12 cells through restoring the lysosomal acidification and recovering the autophgic flux. And these results highlight its therapeutic potential for AD treatment.
Rapamycin and bafilomycin A1 alter autophagy and megakaryopoiesis.[Pubmed:27534900]
Platelets. 2017 Jan;28(1):82-89.
Autophagy is an effective strategy for cell development by recycling cytoplasmic constituents. Genetic deletion of autophagy mediator Atg7 in hematopoietic stem cells (HSCs) can lead to failure of megakaryopoiesis and enhanced autophagy has been implicated in various hematological disorders such as immune thrombocytopenia and myelodysplastic syndrome. Here, we examined the hypothesis that optimal autophagy is essential for megakaryopoiesis and thrombopoiesis by altering autophagy using pharmacological approaches. When autophagy was induced by rapamycin or inhibited by Bafilomycin A1 in fetal liver cells, we observed a significant decrease in high ploidy megakaryocytes, a reduction of CD41 and CD61 co-expressing cells, and less proplatelet or platelet formation. Additionally, reduced cell size was shown in megakaryocytes derived from rapamycin, but not Bafilomycin A1-treated mouse fetal liver cells. However, when autophagy was altered in mature megakaryocytes, we observed no significant change in proplatelet formation, which was consistent with normal platelet counts, megakaryocyte numbers, and ploidy in Atg7(flox/flox) PF4-Cre mice with megakaryocyte- and platelet-specific deletion of autophagy-related gene Atg7. Therefore, our findings suggest that either induction or inhibition of autophagy in the early stage of megakaryopoiesis suppresses megakaryopoiesis and thrombopoiesis.
Bafilomycin A1 induces caspase-independent cell death in hepatocellular carcinoma cells via targeting of autophagy and MAPK pathways.[Pubmed:27845389]
Sci Rep. 2016 Nov 15;6:37052.
Hepatocellular carcinoma (HCC) is refractory to chemotherapies, necessitating novel effective agents. The lysosome inhibitor Bafilomycin A1 (BafA1) at high concentrations displays cytotoxicity in a variety of cancers. Here we show that BafA1 at nanomolar concentrations suppresses HCC cell growth in both 2 dimensional (2D) and 3D cultures. BafA1 induced cell cycle arrest in the G1 phase and triggered Cyclin D1 turnover in HCC cells in a dual-specificity tyrosine phosphorylation-regulated kinase 1B (DYRK1B) dependent manner. Notably, BafA1 induced caspase-independent cell death in HCC cells by impairing autophagy flux as demonstrated by elevated LC3 conversion and p62/SQSTM1 levels. Moreover, genetic ablation of LC3 significantly attenuated BafA1-induced cytotoxicity of HCC cells. We further demonstrate that pharmacological down-regulation or genetic depletion of p38 MAPK decreased BafA1-induced cell death via abolishment of BafA1-induced upregulation of Puma. Notably, knockdown of Puma impaired BafA1-induced HCC cell death, and overexpression of Puma enhanced BafA1-mediated HCC cell death, suggesting a role for Puma in BafA1-mediated cytotoxicity. Interestingly, pharmacological inhibition of JNK with SP600125 enhanced BafA1-mediated cytotoxicity both in vitro and in xenografts derived from HCC cells. Taken together, our data suggest that BafA1 may offer potential as an effective therapy for HCC.
Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes.[Pubmed:16963441]
J Biol Chem. 2006 Nov 24;281(47):36303-16.
Autophagy is a major catabolic pathway in eukaryotic cells whereby the lack of amino acids induces the formation of autophagosomes, double-bilayer membrane vesicles that mediate delivery of cytosolic proteins and organelles for lysosomal degradation. The biogenesis and turnover of autophagosomes in mammalian cells as well as the molecular mechanisms underlying induction of autophagy and trafficking of these vesicles are poorly understood. Here we utilized different autophagic markers to determine the involvement of microtubules in the autophagic process. We show that autophagosomes associate with microtubules and concentrate near the microtubule-organizing center. Moreover, we demonstrate that autophagosomes, but not phagophores, move along these tracks en route for degradation. Disruption of microtubules leads to a significant reduction in the number of mature autophagosomes but does not affect their life span or their fusion with lysosomes. We propose that microtubules serve to deliver only mature autophagosomes for degradation, thus providing a spatial barrier between phagophores and lysosomes.
Chemistry and structure activity relationships of bafilomycin A1, a potent and selective inhibitor of the vacuolar H+-ATPase.[Pubmed:10519916]
Curr Med Chem. 1999 Dec;6(12):1197-212.
Bafilomycin A1, a macrolide antibiotic isolated from the fermentation of Streptomyces spp., is a potent and selective inhibitor of vacuolar-type proton translocating ATP-ases (V-ATPases) and was used to study the physiological role of this class of enzymes. An extensive chemical effort on the unusual structure of this macrolide led to the synthesis of significantly different bafilomycin derivatives. None of the new analogues was more potent than the parent compound but provided a significant amount of information about the structural requirements for the inhibitory activity of Bafilomycin A1 in particular on chicken osteoclast (cOc) ATPase. The vinylic methoxy group adjacent to a carbonyl function, the dienic system and the hydroxy group at position 7 were recognized to be essential features for bafilomycin V-ATPase-inhibitory activity. This information was utilized to design simplified novel derivatives as inhibitors of bone resorption.
Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells.[Pubmed:9639028]
Cell Struct Funct. 1998 Feb;23(1):33-42.
We studied the effects of Bafilomycin A1, a potent and specific inhibitor of vacuolar H+ ATPase (V-ATPase), on the process of autophagy in rat hepatoma cell line, H-4-II-E cells. To induce autophagy, cells were transferred from Dulbecco's modified Eagle medium containing 12% fetal calf serum into Hanks' balanced salt solution. When Bafilomycin A1 was added to Hanks' balanced salt solution, endogenous protein degradation was strongly inhibited and numerous autophagosomes accumulated in H-4-II-E cells, whereas autolysosomes decreased in number. Acid phosphatase activity was not detected in the autophagosomes which accumulated in the presence of Bafilomycin A1, suggesting that fusion between autophagosomes and lysosomes was disturbed by this drug. Inhibition of the fusion was reversible, and the autophagosomes changed into autolysosomes after the removal of the inhibitor. Bafilomycin A1 also prevented the appearance of endocytosed HRP in autophagic vacuoles. These results suggested that acidification of the lumenal space of autophagosomes or lysosomes by V-ATPase is important for the fusion between autophagosomes and lysosomes.


