Bisabolol Oxide ACAS# 22567-36-8 |
Quality Control & MSDS
Number of papers citing our products

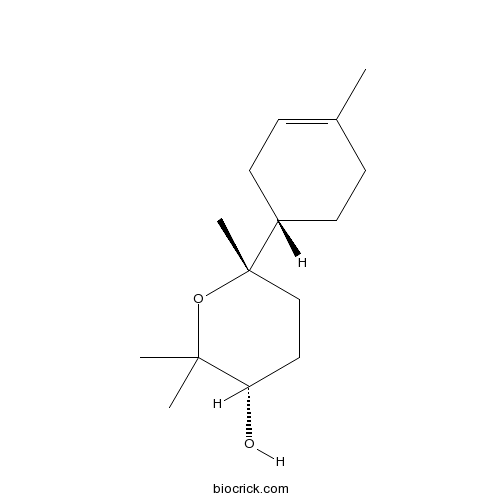
Chemical structure

3D structure
| Cas No. | 22567-36-8 | SDF | Download SDF |
| PubChem ID | 13092559 | Appearance | Pale yellow-brown viscous liquid |
| Formula | C15H26O2 | M.Wt | 238.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | α-Bisabolol oxide; Bisabolol oxide I | ||
| Solubility | Soluble in methan | ||
| Chemical Name | (3S,6S)-2,2,6-trimethyl-6-[(1S)-4-methylcyclohex-3-en-1-yl]oxan-3-ol | ||
| SMILES | CC1=CCC(CC1)C2(CCC(C(O2)(C)C)O)C | ||
| Standard InChIKey | WJHRAVIQWFQMKF-IPYPFGDCSA-N | ||
| Standard InChI | InChI=1S/C15H26O2/c1-11-5-7-12(8-6-11)15(4)10-9-13(16)14(2,3)17-15/h5,12-13,16H,6-10H2,1-4H3/t12-,13+,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bisabololoxide A induces apoptosis in rat thymocytes. |
| In vitro | Bisabololoxide A, one of the main constituents in German chamomile extract, induces apoptosis in rat thymocytes.[Pubmed: 19834689 ]Arch Toxicol. 2010 Jan;84(1):45-52.German chamomile (Matricaria recutita L.), one of the popular ingredients in herbal teas, has been traditionally used for medicinal purposes. Bisabolol oxide A (BSBO) is one of the main constituents in this herb. BSBO is supposed to be principle in some bioactivities of German chamomile such as anti-inflammatory, gastrointestinal, and antipruritic actions. Although the use of German chamomile has spread, the information related to toxicity of BSBO is very limited. Cytotoxic action of bisabololoxide A of German chamomile on human leukemia K562 cells in combination with 5-fluorouracil[Pubmed: 20863677 ]Phytomedicine. 2011 Mar 15;18(5):362-5.German chamomile (Matricaria recutita L.) is a popular ingredient in herbal teas. In previous study, micromolar bisabololoxide A, one of main constituents in German chamomile, exerted cytotoxic action on rat thymocyte, a normal non-proliferative cell. This result prompted us to study the effect of bisabololoxide A on proliferative cancer cells and to seek the possibility of its use with 5-fluorouracil, an anticancer agent.
|

Bisabolol Oxide A Dilution Calculator

Bisabolol Oxide A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1946 mL | 20.9732 mL | 41.9463 mL | 83.8926 mL | 104.8658 mL |
| 5 mM | 0.8389 mL | 4.1946 mL | 8.3893 mL | 16.7785 mL | 20.9732 mL |
| 10 mM | 0.4195 mL | 2.0973 mL | 4.1946 mL | 8.3893 mL | 10.4866 mL |
| 50 mM | 0.0839 mL | 0.4195 mL | 0.8389 mL | 1.6779 mL | 2.0973 mL |
| 100 mM | 0.0419 mL | 0.2097 mL | 0.4195 mL | 0.8389 mL | 1.0487 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isocryptotanshinone
Catalog No.:BCN2499
CAS No.:22550-15-8
- Robustine
Catalog No.:BCN6653
CAS No.:2255-50-7
- Ocotillone
Catalog No.:BCN5066
CAS No.:22549-21-9
- CTU Guanamine
Catalog No.:BCC8921
CAS No.:22535-90-6
- 8-Hydroxy-9,10-diisobutyryloxythymol
Catalog No.:BCN7786
CAS No.:22518-08-7
- Cathepsin Inhibitor 1
Catalog No.:BCC4896
CAS No.:225120-65-0
- Falcarindiol
Catalog No.:BCN5065
CAS No.:225110-25-8
- IDRA 21
Catalog No.:BCC6974
CAS No.:22503-72-6
- N-(1-hydroxy-2-(hydroxymethyl)-4-(4-octylphenyl)butan-2-yl)acetamide
Catalog No.:BCN1483
CAS No.:2249289-10-9
- Erigeside I
Catalog No.:BCN7172
CAS No.:224824-74-2
- Vardenafil HCl Trihydrate
Catalog No.:BCC2277
CAS No.:224785-90-4
- 18-Norabieta-8,11,13-trien-4-ol
Catalog No.:BCN5064
CAS No.:22478-65-5
- Zeorin
Catalog No.:BCN5067
CAS No.:22570-53-2
- Symphytine
Catalog No.:BCN1975
CAS No.:22571-95-5
- PAR 4 (1-6)
Catalog No.:BCC3956
CAS No.:225779-44-2
- Epifriedelanol acetate
Catalog No.:BCN5068
CAS No.:2259-07-6
- Cyclothiazide
Catalog No.:BCC6759
CAS No.:2259-96-3
- (+)-Catechin hydrate
Catalog No.:BCN2309
CAS No.:225937-10-0
- Lucidioline
Catalog No.:BCN7413
CAS No.:22594-91-8
- Auriculine
Catalog No.:BCN2013
CAS No.:22595-00-2
- Demethoxycurcumin
Catalog No.:BCN5974
CAS No.:22608-11-3
- Kulactone
Catalog No.:BCN7953
CAS No.:22611-36-5
- Methyl kulonate
Catalog No.:BCN7952
CAS No.:22611-37-6
- Cinacalcet
Catalog No.:BCC1483
CAS No.:226256-56-0
Inhibition of Fusarium culmorum, Penicillium chrysogenum and Rhizoctonia solani by n-hexane characterized extracts of three plant species as a wood-treated oil-fungicide model.[Pubmed:30887609]
J Appl Microbiol. 2019 Mar 18.
AIMS: Wood as a packing tool is used for packaging and transportation of fruits and vegetables for a period of hours or days. During transportation, fruits or vegetables can be affected by molds with significant postharvest problems. From this way, the present study describes the possibility of using wood-treated oil-fungicide of n-hexane extracts from Eucalyptus camaldulensis (aerial parts), Vitex agenus-castus (leaves) and Matricaria chamomilla (flowers) against the infestation of Fusarium culmorum, Rhizoctonia solani and Penicillium chrysogenum. METHODS AND RESULTS: Air-dried wood samples of Melia azedarach were prepared with the dimensions of 0.5 x 1 x 2 cm and treated with the oily extracts at the concentrations of 0, 1, 2, and 3%. Oils extracted with n-hexane from E. camaldulensis and V. agenus-castus showed promising antifungal activities against the isolated and molecularly identified three fungi F. culmorum, R. solani and P. chrysogenum, while M. chamomilla observed the lowest activity against the studied fungi. GC/MS analysis of oils reported that the major components in E. camaldulensis were beta-fenchol (20.16%), 1,8-cineole (eucalyptol) (12.01%) and sabinene (9.45%); in V. agenus-castus were eucalyptol (44.00%), (E)-beta caryophyllene (13.39%), and beta-sitosterol (12.44%); while in M. chamomilla were Bisabolol Oxide A (16.60%), (Z)-beta-farnesene (16.11%), 4-isopropenyl-1-methyl-cyclohexene (14.18%), and chamazulene (11.27%). CONCLUSIONS: The results suggests using n-hexane oily extracts from E. camaldulensis and V. agenus-castus for wood protection as bio-fungicide. SIGNIFICANCE AND IMPACT OF STUDY: This study highlights the importance of using bio-friendly fungicide agents to protect wood against most common molds occurred during handling of food packaging. The investigated natural oils are represent a source of molecules with potential antifungal activity. This article is protected by copyright. All rights reserved.
Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications.[Pubmed:30172312]
Microbiol Res. 2018 Oct;215:76-88.
Matricaria is a widespread genus of flowering plants of the family Asteraceae that grow in temperate regions of Europe, Asia, America and Africa. Some of the species are also naturalized in Australia. Some species of this genus such as Chamomiles are recognized medicinal plants and cultivated in several countries for commercial purposes: to obtain its blue essence, as herbal tea, and for pharmaceutical or cosmeceutical uses. The phytochemical composition of Matricaria spp. includes volatile terpenoids (e.g., alpha-bisabolol, Bisabolol Oxide A and B, beta-trans-farnesene and chamazulene), sesquiterpene lactones such as matricin, and phenolic compounds (flavonoids, coumarins and phenolic acids). Their essential oil is obtained from the fresh or dried inflorescences by steam distillation, and additionally cohobation of the remaining water. The volatile composition of the essential oil, especially the content of the valuable components alpha-bisabolol and chamazulene, depends on the plant part, origin and quality of the source, genetic, and environmental factors. Moreover, other parameters, such as season of harvest and methods of extraction, can affect the extraction yield of the essential oils/extracts, their composition and, therefore, their bioactivity. Due to the importance of this genus and particularly M. recutita (M. chamomilla), this review focus on its cultivation, factor affecting essential oils' composition and their role in traditional medicine, as antibacterial agents and finally as food preservatives.
Increase of Chamazulene and alpha-Bisabolol Contents of the Essential Oil of German Chamomile (Matricaria chamomilla L.) Using Salicylic Acid Treatments under Normal and Heat Stress Conditions.[Pubmed:28231151]
Foods. 2016 Aug 27;5(3). pii: foods5030056.
The chamazulene and alpha-(-)-bisabolol contents and quality of the chamomile oil are affected by genetic background and environmental conditions. Salicylic acid (SA), as a signaling molecule, plays a significant role in the plant physiological processes. The aim of this study was to evaluate the chemical profile, quantity, and improve the essential oil quality as a consequence of the increase of chamazulene and alpha-(-)-bisabol using salicylic acid under normal and heat stress conditions by the gas chromatography-mass spectrometry (GC-MS) technique. The factorial experiments were carried out during the 2011-2012 hot season using a randomized complete block design with three replications. The factors include four salicylic acid concentrations (0 (control), 10, 25 and 100 mg.L(-1)), and three chamomile cultivars (Bushehr, Bona, Bodegold) were sown on two different planting dates under field conditions. Fourteen compounds were identified from the extracted oil of the samples treated with salicylic acid under normal and heat stress conditions. The major identified oil compositions from chamomile cultivars treated with salicylic acid were chamazulene, alpha-(-)-bisabolol, bisabolone oxide, beta-farnesene, en-yn-dicycloether, and Bisabolol Oxide A and B. Analysis of variance showed that the simple effects (environmental conditions, cultivar and salicylic acid) and their interaction were significant on all identified compounds, but the environmental conditions had no significant effect on Bisabolol Oxide A. The greatest amount of chamazulene obtained was 6.66% at the concentration of 10 mg.L(-1) SA for the Bona cultivar under heat stress conditions, whereas the highest alpha-(-)-bisabolol amount attained was 3.41% at the concentration of 100 mg.L(-1) SA for the Bona cultivar under normal conditions. The results demonstrated that the application of exogenous salicylic acid increases the quantity and essential oil quality as a consequence of the increase of chamazulene and alpha-(-)-bisabolol under normal and heat stress conditions.


