Bonducellpin DCAS# 197781-85-4 |
Quality Control & MSDS
Number of papers citing our products

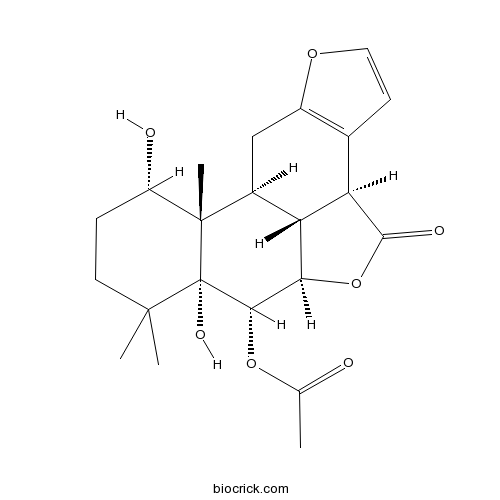
Chemical structure

3D structure
| Cas No. | 197781-85-4 | SDF | Download SDF |
| PubChem ID | 10835061 | Appearance | Powder |
| Formula | C22H28O7 | M.Wt | 404.45 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1S,8S,11R,12S,13R,17S,18S,19R)-13,17-dihydroxy-14,14,18-trimethyl-9-oxo-4,10-dioxapentacyclo[9.7.1.03,7.08,19.013,18]nonadeca-3(7),5-dien-12-yl] acetate | ||
| SMILES | CC(=O)OC1C2C3C(CC4=C(C3C(=O)O2)C=CO4)C5(C1(C(CCC5O)(C)C)O)C | ||
| Standard InChIKey | WIKUZWCBCFNRHH-ZCQRYNMDSA-N | ||
| Standard InChI | InChI=1S/C22H28O7/c1-10(23)28-18-17-16-12(9-13-11(6-8-27-13)15(16)19(25)29-17)21(4)14(24)5-7-20(2,3)22(18,21)26/h6,8,12,14-18,24,26H,5,7,9H2,1-4H3/t12-,14-,15+,16+,17+,18-,21-,22+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Bonducellpin D exhibits moderate activity against four tested human cancer cell lines, HepG-2, K562, HeLa, and Du145. 2. Bonducellpin D may show inhibitory activities on the Para3 virus. |
| Targets | Antifection |

Bonducellpin D Dilution Calculator

Bonducellpin D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4725 mL | 12.3625 mL | 24.7249 mL | 49.4499 mL | 61.8123 mL |
| 5 mM | 0.4945 mL | 2.4725 mL | 4.945 mL | 9.89 mL | 12.3625 mL |
| 10 mM | 0.2472 mL | 1.2362 mL | 2.4725 mL | 4.945 mL | 6.1812 mL |
| 50 mM | 0.0494 mL | 0.2472 mL | 0.4945 mL | 0.989 mL | 1.2362 mL |
| 100 mM | 0.0247 mL | 0.1236 mL | 0.2472 mL | 0.4945 mL | 0.6181 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bonducellpin C
Catalog No.:BCN7647
CAS No.:197781-84-3
- Daphmacrine
Catalog No.:BCN4868
CAS No.:19775-48-5
- Amiodarone HCl
Catalog No.:BCC4377
CAS No.:19774-82-4
- Peimisine
Catalog No.:BCN4992
CAS No.:19773-24-1
- Ajugatakasins A
Catalog No.:BCN3661
CAS No.:197723-20-9
- Loxapine
Catalog No.:BCC4026
CAS No.:1977-10-2
- Mesna
Catalog No.:BCC3811
CAS No.:19767-45-4
- Tigecycline hydrochloride
Catalog No.:BCC4228
CAS No.:197654-04-9
- Fmoc-N-Me-Ser(tBu)-OH
Catalog No.:BCC3353
CAS No.:197632-77-2
- Fmoc-D-β-HomoTrp(Boc)-OH
Catalog No.:BCC2624
CAS No.:197632-75-1
- Fmoc-β-HomoTrp(Boc)-OH
Catalog No.:BCC2625
CAS No.:197632-75-0
- Calystegine B5
Catalog No.:BCN1883
CAS No.:197565-91-6
- 7-O-Acetylbonducellpin C
Catalog No.:BCN7558
CAS No.:197781-86-5
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- SN 003
Catalog No.:BCC7633
CAS No.:197801-88-0
- Stachartin A
Catalog No.:BCN6974
CAS No.:1978388-54-3
- Stachartin B
Catalog No.:BCN6973
CAS No.:1978388-55-4
- Stachartin C
Catalog No.:BCN6972
CAS No.:1978388-56-5
- Stachartin D
Catalog No.:BCN6971
CAS No.:1978388-57-6
- Stachartin E
Catalog No.:BCN6970
CAS No.:1978388-58-7
- GW311616 hydrochloride
Catalog No.:BCC5394
CAS No.:197890-44-1
- AM 404
Catalog No.:BCC6945
CAS No.:198022-70-7
- GW311616
Catalog No.:BCC5393
CAS No.:198062-54-3
- Triptobenzene K
Catalog No.:BCN8055
CAS No.:198129-88-3
Caesalminaxins A-L, cassane diterpenoids from the seeds of Caesalpinia minax.[Pubmed:24303808]
J Nat Prod. 2013 Dec 27;76(12):2210-8.
Fourteen new cassane diterpenoids, caesalminaxins A-L (1-14), and three known compounds were isolated from the seeds of Caesalpinia minax. Among the new diterpenoids, compounds 3 and 4 possess a rare spiro C/D ring system. The C-16 epimeric mixture 1/2 has an unprecedented carbon skeleton, presumably derived from 3 by cleavage of the C-13-C-14 bond. Compound 5 is the first example of a cassane diterpenoid with a spiro A/B ring system. The structures of the compounds were elucidated on the basis of 1D and 2D NMR analysis, and the absolute configurations of 3, 4, 9, and 11 were determined by single-crystal X-ray crystallography. Biosynthesis pathways for 1/2, 3, and 5 are postulated. Compounds 4, 8, and the known Bonducellpin D exhibited moderate activity against four tested human cancer cell lines, HepG-2, K562, HeLa, and Du145.
Molecular structures and antiviral activities of naturally occurring and modified cassane furanoditerpenoids and friedelane triterpenoids from Caesalpinia minax.[Pubmed:11983512]
Bioorg Med Chem. 2002 Jul;10(7):2161-70.
Further investigation of the active components of the chloroform fraction of the seeds of Caesalpinia minax led to the isolation of a new cassane furanoditerpenoid, caesalmin H (1), together with two known furanoditerpenoid lactones, caesalmin B (2) and Bonducellpin D (3). Reduction of the naturally abundant caesalmin D (9), E (10) and F (11) resulted in three new furanoditerpenoid derivatives 4-6. Phytochemical study of the stem of the same plant and subsequent reduction afforded two friedelane triterpenoids (7-8), which were identified by spectroscopic methods. Compounds 1-2 and 4-8 were corroborated by single crystal X-ray analysis. The factors governing the reduction of cassane furanoditerpenoids and friedelane triterpenoids were investigated by correlating the crystallographic results with density functional theory. The inhibitory activities of 2-8 on the Para3 virus were evaluated by cytopathogenic effects (CPE) reduction assay.


