Carfilzomib (PR-171)Proteasome inhibitor,epoxomicin analog CAS# 868540-17-4 |
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- Epoxomicin
Catalog No.:BCC1235
CAS No.:134381-21-8
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
- Gliotoxin
Catalog No.:BCN3894
CAS No.:67-99-2
Quality Control & MSDS
Number of papers citing our products

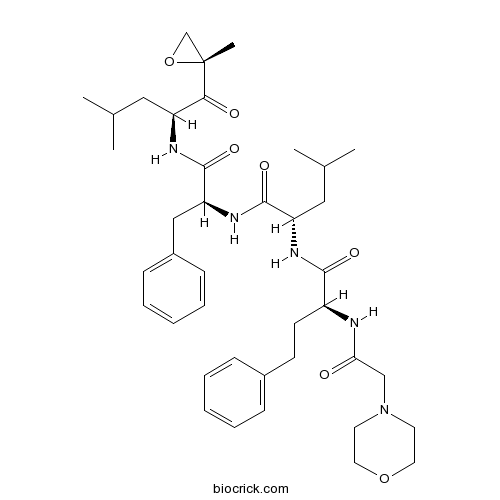
Chemical structure
3D structure
| Cas No. | 868540-17-4 | SDF | Download SDF |
| PubChem ID | 11556711 | Appearance | Powder |
| Formula | C40H57N5O7 | M.Wt | 719.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PR-171 | ||
| Solubility | DMF : ≥ 100 mg/mL (138.91 mM) DMSO : 50 mg/mL (69.45 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S)-4-methyl-N-[(2S)-1-[[(2S)-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]-4-phenylbutanoyl]amino]pentanamide | ||
| SMILES | CC(C)CC(C(=O)C1(CO1)C)NC(=O)C(CC2=CC=CC=C2)NC(=O)C(CC(C)C)NC(=O)C(CCC3=CC=CC=C3)NC(=O)CN4CCOCC4 | ||
| Standard InChIKey | BLMPQMFVWMYDKT-NZTKNTHTSA-N | ||
| Standard InChI | InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Carfilzomib (PR-171) is an irreversible inhibitor of proteasome with IC50 of <5 nM. | |||||
| Targets | Proteasome | |||||
| IC50 | 5 nM | |||||
| Cell experiment [1]: | |
| Cell lines | HT-29 colorectal adenocarcinoma cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 1 h; IC50=9 nM |
| Applications | Incubation of HT-29 colorectal adenocarcinoma cells with PR-171 for 1 h resulted in a dose-dependent inhibition of all three proteasome catalytic activities with the chymotrypsin-like activity exhibiting the greatest sensitivity (IC50=9 nM). The caspase-like and trypsin-like activities were inhibited to a greater extent in the cellular assay (IC50=150–200 nM) than in the isolated enzyme assay (IC50>1 μM). |
| Animal experiment [1]: | |
| Animal models | BNX mice |
| Dosage form | 5 mg/kg delivered weekly; QDx2; intravenous injection |
| Application | The antitumor activity of PR-171 was evaluated in BNX mice bearing established human tumor xenografts derived from three tumor cell lines: HT-29 (colorectal adenocarcinoma), RL (B cell lymphoma ), and HS-Sultan (Burkitt’s lymphoma). All PR-171 dosing schedules (up to 5 mg/kg delivered weekly QDx2) were tolerated in the tumor-bearing animals, resulting in weight loss of <10%. The results show that the activity of PR-171 is dose and schedule dependen. And PR-171 also suppressed proteasome activity in blood and adrenals. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Demo S D, Kirk C J, Aujay M A, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome[J]. Cancer research, 2007, 67(13): 6383-6391. | |

Carfilzomib (PR-171) Dilution Calculator

Carfilzomib (PR-171) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3891 mL | 6.9453 mL | 13.8906 mL | 27.7813 mL | 34.7266 mL |
| 5 mM | 0.2778 mL | 1.3891 mL | 2.7781 mL | 5.5563 mL | 6.9453 mL |
| 10 mM | 0.1389 mL | 0.6945 mL | 1.3891 mL | 2.7781 mL | 3.4727 mL |
| 50 mM | 0.0278 mL | 0.1389 mL | 0.2778 mL | 0.5556 mL | 0.6945 mL |
| 100 mM | 0.0139 mL | 0.0695 mL | 0.1389 mL | 0.2778 mL | 0.3473 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
Carfilzomib has been review as a new agent to treat relapsed and refractory MM.
Abstract
Instead of inhibiting the formation of OC sealing zone alone, the combination of cartfilzomib and CC-292 exhibited synergistic anti-MM activities, including inhibition of both sealing zone formation and differentiation of OC, suppression of tumor burden in a mouse model and increasing bone volume.
Abstract
Carfilzomib, a proteasome inhibitor, is an anti-MCL agent that concentration-dependently inhibited cell growth, induced apoptosis and suppressed survival signaling pathways NF-KB and STAT3 without causing toxicity to normal peripheral blood mononuclear cells. Immunoproteasome, particularly LMP2, plays an indispensible role in anti-MCL activity of carfilzomib.
Abstract
Even though it is an FDA-approved anti-MM drug with less than 1% TLS frequency, carfilzomib has been associated with TLS development in a 55-year-old male patient with relapsed MM.
Abstract
Carfilzomib, an FDA-approved anti-MM drug, has been evaluated in the treatment of patients who have relapsed and refractory MM and received prior bortezomib and thalidomide or lenalidomide.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
An epoxomicin derivate with potential antineoplastic activity. It irreversibly binds to and inhibits the chymotrypsin-like activity of the 20S proteasome, an enzyme responsible for degrading a large variety of cellular proteins. Inhibition of proteasome-mediated proteolysis results in an accumulation of polyubiquinated proteins, which may lead to cell cycle arrest, induction of apoptosis, and inhibition of tumor growth.
References:
1. Guido Cavaletti, et al., Leukemia & Lymphoma (2010), 51(7), 1178-1187.
2. Girija Dasmahapatra, et al., Blood (2010), 115(22), 4478-4487.
- Iristectorigenin B
Catalog No.:BCN8391
CAS No.:39012-01-6
- (+)-Puerol B 2''-O-glucoside
Catalog No.:BCN4561
CAS No.:868409-19-2
- Protosappanin A dimethyl acetal
Catalog No.:BCN6517
CAS No.:868405-37-2
- 2,2,5,5-Tetramethylcyclohexane-1,4-dione
Catalog No.:BCN1324
CAS No.:86838-54-2
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- Rhodiosin; Herbacetin-7-O-glucorhamnoside
Catalog No.:BCN8478
CAS No.:86831-54-1
- Rhodiolin
Catalog No.:BCC8356
CAS No.:86831-53-0
- Org 27569
Catalog No.:BCC4411
CAS No.:868273-06-7
- LY 2365109 hydrochloride
Catalog No.:BCC7677
CAS No.:868265-28-5
- Pam2CSK4
Catalog No.:BCC6247
CAS No.:868247-72-7
- SID 7969543
Catalog No.:BCC6026
CAS No.:868224-64-0
- Carasiphenol C
Catalog No.:BCN8251
CAS No.:868168-04-1
- 3',5'-Dimethoxybiphenyl-3-ol
Catalog No.:BCN7529
CAS No.:868666-20-0
- (R)-(-)-α-Methylhistamine dihydrobromide
Catalog No.:BCC5665
CAS No.:868698-49-1
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
- Eudesma-3,11-dien-2-one
Catalog No.:BCN7607
CAS No.:86917-79-5
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
- PF-573228
Catalog No.:BCC4496
CAS No.:869288-64-2
- Neurokinin A (porcine)
Catalog No.:BCC6955
CAS No.:86933-74-6
- Neurokinin B (human, porcine)
Catalog No.:BCC7119
CAS No.:86933-75-7
- AZD8330
Catalog No.:BCC3733
CAS No.:869357-68-6
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- 14-Deoxy-17-hydroxyandrographolide
Catalog No.:BCN4560
CAS No.:869384-82-7
- Praeroside II
Catalog No.:BCN7001
CAS No.:86940-46-7
A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies.[Pubmed:19903785]
Clin Cancer Res. 2009 Nov 15;15(22):7085-91.
PURPOSE: Carfilzomib (formerly PR-171) is a novel proteasome inhibitor of the epoxyketone class that is selective and structurally distinct from bortezomib. Proteasome inhibition by carfilzomib is mechanistically irreversible. Consequently, proteasome inhibition is more sustained with carfilzomib than with bortezomib. EXPERIMENTAL DESIGN: In a phase 1 trial evaluating the safety and efficacy of carfilzomib in relapsed or refractory hematologic malignancies, eight dose groups of three to six patients received 5 consecutive days of carfilzomib i.v. push at doses of 1.2, 2.4, 4, 6, 8.4, 11, 15, and 20 mg/m2 within 14-day cycles. RESULTS: Twenty-nine patients enrolled that were relapsed or refractory after at least two prior therapies. Nonhematologic toxicities included fatigue, nausea, and diarrhea in more than one third of patients-mostly grade 1 or 2 in severity. At 20 mg/m2, grade 3 febrile neutropenia and grade 4 thrombocytopenia were reported, establishing 15 mg/m2 as the maximum tolerated dose. No grade 3 or 4 peripheral neuropathies were reported. Antitumor activity was observed at doses > or =11 mg/m2: one unconfirmed complete response (mantle cell), one partial response (multiple myeloma), and two minimal responses (multiple myeloma and Waldenstrom's macroglobulinemia). CONCLUSION: This is the first clinical use of carfilzomib that shows tolerability and clinical activity in multiple hematologic malignancies using consecutive-day dosing.
Phase 1 trial of carfilzomib (PR-171) in combination with vorinostat (SAHA) in patients with relapsed or refractory B-cell lymphomas.[Pubmed:26284612]
Leuk Lymphoma. 2016;57(3):635-43.
A phase 1 study with carfilzomib and vorinostat was conducted in 20 B-cell lymphoma patients. Vorinostat was given orally twice daily on days 1, 2, 3, 8, 9, 10, 15, 16, and 17 followed by carfilzomib (given as a 30-min infusion) on days 1, 2, 8, 9, 15, and 16. A treatment cycle was 28 days. Dose escalation initially followed a standard 3 + 3 design, but adapted a more conservative accrual rule following dose de-escalation. The maximum tolerated dose was 20 mg/m2 carfilzomib and 100 mg vorinostat (twice daily). The dose-limiting toxicities were grade 3 pneumonitis, hyponatremia, and febrile neutropenia. One patient had a partial response and two patients had stable disease. Correlative studies showed a decrease in NF-kappaB activation and an increase in Bim levels in some patients, but these changes did not correlate with clinical response.


