Chamaejasmenin ACAS# 89595-71-1 |
- 4',4'''-Di-O-methylisochamaejasmin
Catalog No.:BCN6849
CAS No.:1620921-68-7
- Isochamaejasmenin B
Catalog No.:BCN3045
CAS No.:865852-48-8
Quality Control & MSDS
Number of papers citing our products

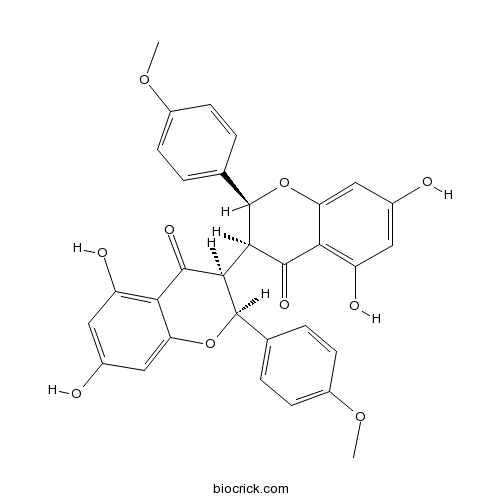
Chemical structure

3D structure
| Cas No. | 89595-71-1 | SDF | Download SDF |
| PubChem ID | 21676273 | Appearance | Powder |
| Formula | C32H26O10 | M.Wt | 570.6 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3S)-3-[(2S,3S)-5,7-dihydroxy-2-(4-methoxyphenyl)-4-oxo-2,3-dihydrochromen-3-yl]-5,7-dihydroxy-2-(4-methoxyphenyl)-2,3-dihydrochromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2C(C(=O)C3=C(C=C(C=C3O2)O)O)C4C(OC5=CC(=CC(=C5C4=O)O)O)C6=CC=C(C=C6)OC | ||
| Standard InChIKey | BTCICADMSGBCKA-QWWQXMGCSA-N | ||
| Standard InChI | InChI=1S/C32H26O10/c1-39-19-7-3-15(4-8-19)31-27(29(37)25-21(35)11-17(33)13-23(25)41-31)28-30(38)26-22(36)12-18(34)14-24(26)42-32(28)16-5-9-20(40-2)10-6-16/h3-14,27-28,31-36H,1-2H3/t27-,28-,31-,32-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Chamaejasmenin A Dilution Calculator

Chamaejasmenin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7525 mL | 8.7627 mL | 17.5254 mL | 35.0508 mL | 43.8135 mL |
| 5 mM | 0.3505 mL | 1.7525 mL | 3.5051 mL | 7.0102 mL | 8.7627 mL |
| 10 mM | 0.1753 mL | 0.8763 mL | 1.7525 mL | 3.5051 mL | 4.3814 mL |
| 50 mM | 0.0351 mL | 0.1753 mL | 0.3505 mL | 0.701 mL | 0.8763 mL |
| 100 mM | 0.0175 mL | 0.0876 mL | 0.1753 mL | 0.3505 mL | 0.4381 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chamaejasmenin C
Catalog No.:BCN3043
CAS No.:89595-70-0
- Mogroside VI
Catalog No.:BCN2578
CAS No.:89590-98-7
- Mogroside IV
Catalog No.:BCN2532
CAS No.:89590-95-4
- Flumatinib mesylate
Catalog No.:BCC3970
CAS No.:895519-91-2
- SC 144
Catalog No.:BCC1171
CAS No.:895158-95-9
- Methylneoquassin
Catalog No.:BCN3121
CAS No.:89498-93-1
- Picrasinol B
Catalog No.:BCN4440
CAS No.:89498-91-9
- TCS JNK 6o
Catalog No.:BCC7607
CAS No.:894804-07-0
- ST 2825
Catalog No.:BCC1967
CAS No.:894787-30-5
- BIBF 1202
Catalog No.:BCC5298
CAS No.:894783-71-2
- DMOG
Catalog No.:BCC2433
CAS No.:89464-63-1
- [D-Trp7,9,10]-Substance P
Catalog No.:BCC7202
CAS No.:89430-38-6
- 2-Acetamidoethyl phosphate
Catalog No.:BCN1760
CAS No.:89603-45-2
- Imiquimod maleate
Catalog No.:BCC4197
CAS No.:896106-16-4
- trans-3-Oxo-alpha-ionol
Catalog No.:BCN3385
CAS No.:896107-70-3
- AT9283
Catalog No.:BCC2173
CAS No.:896466-04-9
- BMH-21
Catalog No.:BCC5580
CAS No.:896705-16-1
- VX-11e
Catalog No.:BCC2051
CAS No.:896720-20-0
- Androstadienedione
Catalog No.:BCC8824
CAS No.:897-06-3
- KX2-391
Catalog No.:BCC5080
CAS No.:897016-82-9
- 7-O-Acetyl-4-O-demethylpolysyphorin
Catalog No.:BCN3984
CAS No.:89706-39-8
- AST-1306
Catalog No.:BCC3727
CAS No.:897383-62-9
- Toremifene
Catalog No.:BCC2010
CAS No.:89778-26-7
- Toremifene Citrate
Catalog No.:BCC4487
CAS No.:89778-27-8
In vitro anti-cancer activity of chamaejasmenin B and neochamaejasmin C isolated from the root of Stellera chamaejasme L.[Pubmed:23222270]
Acta Pharmacol Sin. 2013 Feb;34(2):262-70.
AIM: To examine the anti-cancer effects of chamaejasmenin B and neochamaejasmin C, two biflavonones isolated from the root of Stellera chamaejasme L (known as the traditional Chinese herb Rui Xiang Lang Du) in vitro. METHODS: Human liver carcinoma cell lines (HepG2 and SMMC-7721), a human non-small cell lung cancer cell line (A549), human osteosarcoma cell lines (MG63, U2OS, and KHOS), a human colon cancer cell line (HCT-116) and a human cervical cancer cell line (HeLa) were used. The anti-proliferative effects of the compounds were measured using SRB cytotoxicity assay. DNA damage was detected by immunofluorescence and Western blotting. Apoptosis and cell cycle distribution were assessed using flow cytometry analysis. The expression of the related proteins was examined with Western blotting analysis. RESULTS: Both chamaejasmenin B and neochamaejasmin C exerted potent anti-proliferative effects in the 8 human solid tumor cell lines. Chamaejasmenin B (the IC(50) values ranged from 1.08 to 10.8 mumol/L) was slightly more potent than neochamaejasmin C (the IC(50) values ranged from 3.07 to 15.97 mumol/L). In the most sensitive A549 and KHOS cells, the mechanisms underlying the anti-proliferative effects were characterized. The two compounds induced prominent expression of the DNA damage marker gamma-H2AX as well as apoptosis. Furthermore, treatment of the cells with the two compounds caused prominent G(0)/G(1) phase arrest. CONCLUSION: Chamaejasmenin B and neochamaejasmin C are potential anti-proliferative agents in 8 human solid tumor cell lines in vitro via inducing cell cycle arrest, apoptosis and DNA damage.
Chamaejasmenin B, a novel candidate, inhibits breast tumor metastasis by rebalancing TGF-beta paradox.[Pubmed:27374079]
Oncotarget. 2016 Jul 26;7(30):48180-48192.
Metastasis is the leading lethal factor severely restraining the effectiveness of clinical treatment. TGF-beta is the key regulator for metastasis and influences paradoxically on cancer progression. The known TGF-beta blockers exert little selectivity on its functions, indiscriminately causing the anti-metastatic and pro-growth effects. Under such circumstances, specifically rebalancing the oncological function of TGF-beta provides a crucial oncotarget against metastasis. In our study, we established the screening platform targeting cell motility and identified a potential flavonoid, Chamaejasmenin B (ICJ), extracted from Stellera chamaejasme L..It suppressed the migration and invasion in breast cancer cells in vitro. Moreover, by dynamical quantification of breast cancer progression in small-animal imaging system, ICJ was proved to be a potent inhibitor of metastasis with minimal toxic side effects. Mechanism study further revealed that ICJ efficiently blocked TGF-beta induced EMT, disrupted the interaction between beta3 integrin-TbetaRII complex and, consequently, resulted in the selective inhibition of FAK:Src:p38 pathway. Meanwhile, specific blockage of this pathway largely attenuated the anti-metastatic function of ICJ. Importantly, in contrast with the antagonistic effects on TGF-beta induced metastasis, ICJ obviously sensitized its cytostatic activity, suggesting that it was not a pan-blocker but a rebalancer for the functional output of TGF-beta. Collectively, by targeting TGF-beta Paradox, we experimentally provided a promising candidate for metastatic intervention.


