Cichoric acidCAS# 6537-80-0 |
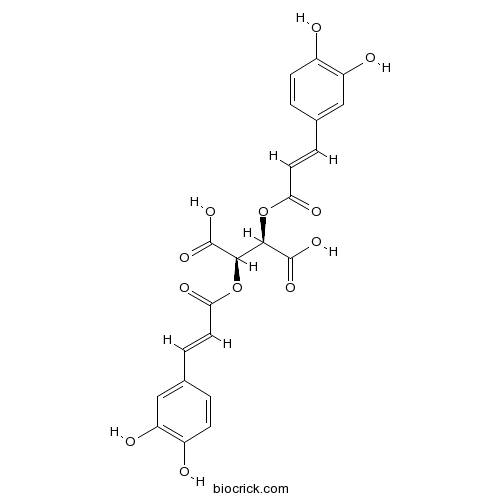
- D-Chicoric Acid
Catalog No.:BCC8148
CAS No.:52248-48-3
- Chicoric acid
Catalog No.:BCN1215
CAS No.:70831-56-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 6537-80-0 | SDF | Download SDF |
| PubChem ID | 5281764 | Appearance | Powder |
| Formula | C22H18O12 | M.Wt | 474.37 |
| Type of Compound | Other Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | dicaffeoyltartaric acid | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R)-2,3-bis[[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy]butanedioic acid | ||
| SMILES | C1=CC(=C(C=C1C=CC(=O)OC(C(C(=O)O)OC(=O)C=CC2=CC(=C(C=C2)O)O)C(=O)O)O)O | ||
| Standard InChIKey | YDDGKXBLOXEEMN-IABMMNSOSA-N | ||
| Standard InChI | InChI=1S/C22H18O12/c23-13-5-1-11(9-15(13)25)3-7-17(27)33-19(21(29)30)20(22(31)32)34-18(28)8-4-12-2-6-14(24)16(26)10-12/h1-10,19-20,23-26H,(H,29,30)(H,31,32)/b7-3+,8-4+/t19-,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cichoric acid Dilution Calculator

Cichoric acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1081 mL | 10.5403 mL | 21.0806 mL | 42.1612 mL | 52.7015 mL |
| 5 mM | 0.4216 mL | 2.1081 mL | 4.2161 mL | 8.4322 mL | 10.5403 mL |
| 10 mM | 0.2108 mL | 1.054 mL | 2.1081 mL | 4.2161 mL | 5.2701 mL |
| 50 mM | 0.0422 mL | 0.2108 mL | 0.4216 mL | 0.8432 mL | 1.054 mL |
| 100 mM | 0.0211 mL | 0.1054 mL | 0.2108 mL | 0.4216 mL | 0.527 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Anethole, trans-
Catalog No.:BCN9032
CAS No.:4180-23-8
- Galactaric acid
Catalog No.:BCN9031
CAS No.:526-99-8
- (±)-Nicotine
Catalog No.:BCN9030
CAS No.:22083-74-5
- Cynatratoside C
Catalog No.:BCN9029
CAS No.:
- Byakangelicin
Catalog No.:BCN9028
CAS No.:482-25-7
- Neolancerin
Catalog No.:BCN9027
CAS No.:117221-65-5
- (-)-Carvone
Catalog No.:BCN8949
CAS No.:6485-40-1
- Cyanidin-3-O-(6''-malonylglucoside) chloride
Catalog No.:BCN9026
CAS No.:171828-62-9
- Sipeimine-3-beta-D-glucoside
Catalog No.:BCN9025
CAS No.:67968-40-5
- Robinetinidin chloride
Catalog No.:BCN9024
CAS No.:3020-09-5
- (+)-Mediresinol Di-O-beta-D-glucopyranoside
Catalog No.:BCN9023
CAS No.:88142-63-6
- Peonidin-3,5-O-diglucoside chloride
Catalog No.:BCN9022
CAS No.:132-37-6
- Isopongachromene
Catalog No.:BCN9034
CAS No.:
- 1-Naphthaleneacetic acid
Catalog No.:BCN9035
CAS No.:86-87-3
- Kizuta saponin K11
Catalog No.:BCN9036
CAS No.:97240-03-4
- D-(+)-Lactose Monohydrate
Catalog No.:BCN9037
CAS No.:64044-51-5
- Ganoderic acid E
Catalog No.:BCN9038
CAS No.:
- Fluoranthene
Catalog No.:BCN9039
CAS No.:206-44-0
- DL-α-Tocopherol
Catalog No.:BCN9040
CAS No.:10191-41-0
- Peimisine hydrochloride
Catalog No.:BCN9041
CAS No.:900498-44-4
- (+)-Secoisolariciresinol
Catalog No.:BCN9042
CAS No.:145265-02-7
- Allocryptopine
Catalog No.:BCN9043
CAS No.:485-91-6
- Quininic acid
Catalog No.:BCN9044
CAS No.:86-68-0
- Menthol
Catalog No.:BCN9045
CAS No.:89-78-1
Effect of Shading on Development, Yield and Quality of Bastard Balm Herb (Melittis melissophyllum L.).[Pubmed:32375290]
Molecules. 2020 May 3;25(9). pii: molecules25092142.
The aim of the study was to assess the effects of Melittis melissophyllum shading on its development and accumulation of phenolics. Their content (verbascoside, apiin, luteolin-7-O-glucoside, coumarin, 3,4-dihydroxycoumarin, o-coumaric acid 2-O-glucoside as well as o-coumaric, p-coumaric, chlorogenic, caffeic, ferulic and Cichoric acid) was determined in the herb using HPLC-DAD. The results showed that the content of abovementioned flavonoids and phenolic acids was highest in plants grown under full sunlight. On the other hand, a higher content of coumarin was observed in shaded plants, especially after the seed-setting stage. A similar tendency was noted for the amount of chlorophyll a and b. The content of hydrogen peroxide and malondialdehyde, the activity of polyphenol oxidase and catalase and the antioxidant capacity of plant extracts (measured using DPPH, ABTS and FRAP assays) were found to be the highest in the plants grown in full sunlight. However, the plants grown in moderate (30%) shade were found to thrive best.
Cichoric acid attenuates the toxicity of mesotrione. Effect on in vitro skin cell model.[Pubmed:32279013]
Environ Toxicol Pharmacol. 2020 Jul;77:103375.
There is an important need to increase knowledge regarding the interactions between environmental contaminants and other compounds. Pesticides are an important group of food contaminants. By contrast, Cichoric acid (CA) belongs to the category of desirable food ingredients with antioxidant and cytotoxic effects. The aim of the presented study was to test if CA may constitute a food ingredient, which eliminate stimulatory effect of pesticides on skin cancer cells and toxic effect of herbicides on fibroblasts. Therefore, we conducted cytotoxicity studies of environmentally relevant pesticide concentrations and the mixture of both compounds in melanoma and fibroblasts cells. We studied if CA combined with mesotrione change the oxidative stress parameters and apoptotic activity in treated cells. Obtained results indicate that CA exhibits cytotoxic activity against mesotrione-induced skin cancer development by influencing oxidative stress parameters and apoptosis. On the other hand CA inhibits prooxidative and proapoptotic activity of mesotrione in fibroblasts. Presented methods and obtained results could be a useful tool in the analysis of environmental contaminants toxicity and possible preventive activity of antioxidative plant- origin compounds.
Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes.[Pubmed:32218153]
Antioxidants (Basel). 2020 Mar 25;9(4). pii: antiox9040272.
Selenium (Se) is considered essential for human nutrition as it is involved in the metabolic pathway of selenoproteins and relevant biological functions. Microgreens, defined as tender immature greens, constitute an emerging functional food characterized by overall higher levels of phytonutrients than their mature counterparts. The nutraceutical value of microgreens can be further improved through Se biofortification, delivering Se-enriched foods and potentially an enhanced content of bioactive compounds. The current study defined the effect of sodium selenate applications at three concentrations (0, 8, and 16 muM Se) on the bioactive compounds and mineral content of coriander, green basil, purple basil, and tatsoi microgreens grown in soilless cultivation. Analytical emphasis was dedicated to the identification and quantification of polyphenols by UHPLC-Q-Orbitrap-HRMS, major carotenoids by HPLC-DAD, and macro micro-minerals by ICP-OES. Twenty-seven phenolic compounds were quantified, of which the most abundant were: Chlorogenic acid and rutin in coriander, caffeic acid hexoside and kaempferol-3-O(caffeoyl) sophoroside-7-O-glucoside in tatsoi, and Cichoric acid and rosmarinic acid in both green and purple basil. In coriander and tatsoi microgreens, the application of 16 muM Se increased the total phenols content by 21% and 95%, respectively; moreover, it improved the yield by 44% and 18%, respectively. At the same Se dose, the bioactive value of coriander and tatsoi was enhanced by a significant increase in rutin (33%) and kaempferol-3-O(feruloyl)sophoroside-7-O-glucoside (157%), respectively, compared to the control. In green and purple basil microgreens, the 8 muM Se application enhanced the lutein concentration by 7% and 19%, respectively. The same application rate also increased the overall macroelements content by 35% and total polyphenols concentration by 32% but only in the green cultivar. The latter actually had a tripled chicoric acid content compared to the untreated control. All microgreen genotypes exhibited an increase in the Se content in response to the biofortification treatments, thereby satisfying the recommended daily allowance for Se (RDA-Se) from 20% to 133%. The optimal Se dose that guarantees the effectiveness of Se biofortification and improves the content of bioactive compounds was 16 muM in coriander and tatsoi, and 8 muM in green and purple basil.
Biological Activity of New Cichoric Acid-Metal Complexes in Bacterial Strains, Yeast-Like Fungi, and Human Cell Cultures In Vitro.[Pubmed:31935840]
Nutrients. 2020 Jan 6;12(1). pii: nu12010154.
Cichoric acid (CA) belongs to the group of polyphenols, which occurs in a variety of plant species and it is characterized by anticancer, antibacterial, and antiviral properties. Selected polyphenols have the ability to combine with metal ions to form chelate complexes that reveal greater biological activity than free compounds. In order to study possible antimicrobial and anticancer effect of CA and its complexes with copper(II)/zinc(II)/nickel(II)/cobalt(II) we decided to conduct cytotoxicity tests to estimate the most effective concentrations of tested compounds. The results of the presented study demonstrated, for the first time, that the treatment with newly synthesized CA-metal complexes has anticancer and antimicrobial effects, which were examined in seven different cell lines: MCF-7, MDA-MB-231, and ZR-75-1 breast cancer cell lines, A375 melanoma cell line, DLD-1 cell line, LN-229 cell line, FN cell line; five bacterial strains: Escherichia coli, Pseudomonas aeruginosa, Staphylococcus epidermidis, Proteus vulgaris, Lactobacillus rhamnosus, yeast Sacchcaromyces boulardii, and pathogenic yeast-like fungi Candida albicans. The presented study indicates that CA-metal complexes could be considered as a potential supplementary tool in anticancer therapy, however, because of their possible toxic activity on fibroblasts, they should be used with caution. Some of the tested complexes have also preservative properties and positive influence on normal non-pathogenic microorganisms, which was demonstrated in selected microbial strains, therefore they may serve as food preservatives of natural origin with cytoprotective properties.


