Compound 56REGFR inhibitor CAS# 171745-13-4 |
- AG-18
Catalog No.:BCC1051
CAS No.:118409-57-7
- Icotinib Hydrochloride
Catalog No.:BCC1639
CAS No.:1204313-51-8
- AZD-9291
Catalog No.:BCC4120
CAS No.:1421373-65-0
- Gefitinib hydrochloride
Catalog No.:BCC1591
CAS No.:184475-55-6
- Pelitinib (EKB-569)
Catalog No.:BCC1118
CAS No.:257933-82-7
- AZD8931 (Sapitinib)
Catalog No.:BCC3734
CAS No.:848942-61-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure
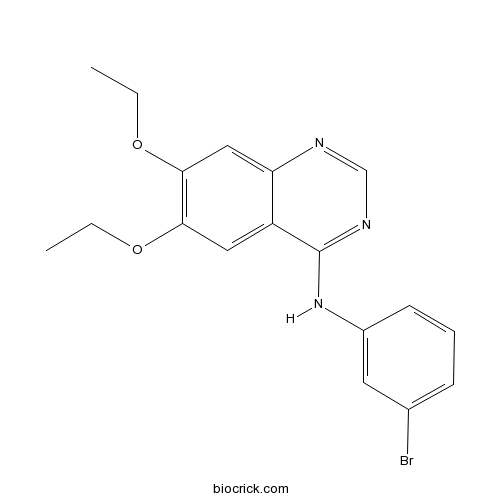
3D structure
| Cas No. | 171745-13-4 | SDF | Download SDF |
| PubChem ID | 2857 | Appearance | Powder |
| Formula | C18H18BrN3O2 | M.Wt | 388.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >16.95mg/mL in DMSO | ||
| Chemical Name | N-(3-bromophenyl)-6,7-diethoxyquinazolin-4-amine | ||
| SMILES | CCOC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC(=CC=C3)Br)OCC | ||
| Standard InChIKey | YXOXHAUUTIOBDA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H18BrN3O2/c1-3-23-16-9-14-15(10-17(16)24-4-2)20-11-21-18(14)22-13-7-5-6-12(19)8-13/h5-11H,3-4H2,1-2H3,(H,20,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Compound 56 is a cell-permeable, reversible, and ATP-competitive inhibitor of tyrosine kinase activity of EGFR. | |||||
| Targets | EGFR | |||||

Compound 56 Dilution Calculator

Compound 56 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5753 mL | 12.8766 mL | 25.7533 mL | 51.5066 mL | 64.3832 mL |
| 5 mM | 0.5151 mL | 2.5753 mL | 5.1507 mL | 10.3013 mL | 12.8766 mL |
| 10 mM | 0.2575 mL | 1.2877 mL | 2.5753 mL | 5.1507 mL | 6.4383 mL |
| 50 mM | 0.0515 mL | 0.2575 mL | 0.5151 mL | 1.0301 mL | 1.2877 mL |
| 100 mM | 0.0258 mL | 0.1288 mL | 0.2575 mL | 0.5151 mL | 0.6438 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Compound 56, 4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline, is a potent and specific inhibitor of the tyrosine kinase of the epidermal growth factor receptor (EGFR) showing an IC50 of 0.006 nM. It competitively binds at the adenosine-triphosphate (ATP) site of EGFR. Compound 56 is capable of inhibiting the phosphorylation of EGF-dependent EGFR, suppressing the proliferation and clonogenicity of a wide panel of EGFR-overexpressing human cancer lines, and blocking EGF-mediated mitogenesis and oncogenic transformation in fibroblasts overexpressing EGFR. Besides inhibiting EGFR tyrosine kinase, It also inhibits the tyrosine kinase of human epidermal growth factor receptor 2 (HER2/neu) but with a less potency.
Reference
Bridges AJ, Zhou H, Cody DR, Rewcastle GW, McMichael A, Showalter HD, Fry DW, Kraker AJ, and Denny WA. Tyrosine kinase inhibitors. 8. An unusually steep structure-activity relationship for analogues of 4-(3-bromoanilino)-6,7-dimethoxyquinazoline (PD 153035), a potent inhibitor of the epidermal growth factor receptor.J Med Chem 1966; 39 (1): 267-276
Monique Bos, Jhn Mendelsohn, Young-Mee Kim, Joan Albanell, David W. Fry, and Jose Baelga. PD153035, a tyrosine kinase inhibitor, prevents epidermal growth factor receptor activation and inhibits growth of cancer cells in a receptor number-dependent manner. Clin Cancer Res 1997;3:2099-2106
- Nitidanin
Catalog No.:BCN1107
CAS No.:171674-89-8
- Sodium Phenylbutyrate
Catalog No.:BCC2164
CAS No.:1716-12-7
- Sildenafil Citrate
Catalog No.:BCC2276
CAS No.:171599-83-0
- NBI-98854
Catalog No.:BCC4278
CAS No.:171598-74-6
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Tadalafil
Catalog No.:BCC2281
CAS No.:171596-29-5
- Doederleinic Acid
Catalog No.:BCC8319
CAS No.:171596-14-8
- 10,11-Dihydro-24-hydroxyaflavinine
Catalog No.:BCN7440
CAS No.:171569-81-6
- Urocortin (rat)
Catalog No.:BCC5789
CAS No.:171543-83-2
- Alexidine dihydrochloride
Catalog No.:BCC2466
CAS No.:1715-30-6
- Myrislignan
Catalog No.:BCN1242
CAS No.:171485-39-5
- Salprionin
Catalog No.:BCN3162
CAS No.:171439-43-3
- S-Adenosyl-L-Methtonine
Catalog No.:BCN2231
CAS No.:17176-17-9
- 2-(1H-Indole-3-carboxamido)benzoic acid
Catalog No.:BCN1529
CAS No.:171817-95-1
- Euchrenone A10
Catalog No.:BCN3574
CAS No.:171828-81-2
- 17-Acetyloxy-6-chloro-1α-chloromethylpregna-4,6-diene-3,20-dione
Catalog No.:BCC8440
CAS No.:17183-98-1
- Cauloside A
Catalog No.:BCN6726
CAS No.:17184-21-3
- 3-O-Coumaroylarjunolic acid
Catalog No.:BCN7131
CAS No.:171864-20-3
- Tryprostatin A
Catalog No.:BCN6778
CAS No.:171864-80-5
- Palmatine chloride monohydrate
Catalog No.:BCC8228
CAS No.:171869-95-7
- SCH 51344
Catalog No.:BCC5613
CAS No.:171927-40-5
- H-Phe(4-NO2)-OMe.HCl
Catalog No.:BCC3294
CAS No.:17193-40-7
- Alpha-Tocotrienol
Catalog No.:BCN3724
CAS No.:1721-51-3
- Gericudranin E
Catalog No.:BCN8070
CAS No.:172104-07-3
The analysis of microsatellites and compound microsatellites in 56 complete genomes of Herpesvirales.[Pubmed:25172209]
Gene. 2014 Nov 1;551(1):103-9.
Simple sequence repeats (SSRs), or microsatellites, are special DNA/RNA sequences with repeated unit of 1-6 bp. The genomes of Herpesvirales have many repeating structures, which is an excellent system to study the evolution and roles of microsatellites and compound microsatellites in viruses. Therefore, 56 genomes of Herpesvirales were selected and the occurrence, composition and complexity of different repeats were investigated in the genomes. A total of 63,939 microsatellites and 5825 compound microsatellites were extracted from 56 genomes. It found that GC content has a significant strong correlation with both the counts of microsatellites (CM) and the counts of compound microsatellites (CCM). However, genome size has a moderate correlation only with CM and almost no correlation with CCM. The compound microsatellites occurring in genic regions are obviously more than that in intergenic regions. In general, the number of compound microsatellite decreases with the increase of complexity (C) (the count of individual microsatellites being part of a compound microsatellite) and the complexity hardly exceeds C=4. The vast majority of compound microsatellites exist in intergenic regions, when C>/=10. The distributions of SSRs tend to be organism-specific rather than host-specific in herpesvirus genomes. The diversity of microsatellites and compound microsatellites may be helpful for a better understanding of the viral genetic diversity, genotyping, and evolutionary biology in herpesviruses genomes.
Magnetic Properties of a Single-Molecule Lanthanide-Transition-Metal Compound Containing 52 Gadolinium and 56 Nickel Atoms.[Pubmed:26923173]
Angew Chem Int Ed Engl. 2016 Mar 24;55(14):4532-6.
Monodisperse metal clusters provide a unique platform for investigating magnetic exchange within molecular magnets. Herein, the core-shell structure of the monodisperse molecule magnet of [Gd52 Ni56 (IDA)48 (OH)154 (H2 O)38 ]@SiO2 (1 a@SiO2 ) was prepared by encapsulating one high-nuclearity lanthanide-transition-metal compound of [Gd52 Ni56 (IDA)48 (OH)154 (H2 O)38 ](NO3 )18 164 H2 O (1) (IDA=iminodiacetate) into one silica nanosphere through a facile one-pot microemulsion method. 1 a@SiO2 was characterized using transmission electron microscopy, N2 adsorption-desorption isotherms, and inductively coupled plasma-atomic emission spectrometry. Magnetic investigation of 1 and 1 a revealed J1 =0.25 cm(-1) , J2 =-0.060 cm(-1) , J3 =-0.22 cm(-1) , J4 =-8.63 cm(-1) , g=1.95, and z J=-2.0x10(-3) cm(-1) for 1, and J1 =0.26 cm(-1) , J2 =-0.065 cm(-1) , J3 =-0.23 cm(-1) , J4 =-8.40 cm(-1) g=1.99, and z J=0.000 cm(-1) for 1 a@SiO2 . The z J=0 in 1 a@SiO2 suggests that weak antiferromagnetic coupling between the compounds is shielded by silica nanospheres.


