Cucurbitacin ISTAT3/JAK2 signaling inhibitor CAS# 2222-07-3 |
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- Bardoxolone methyl
Catalog No.:BCC1400
CAS No.:218600-53-4
- Ruxolitinib (INCB018424)
Catalog No.:BCC1276
CAS No.:941678-49-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure
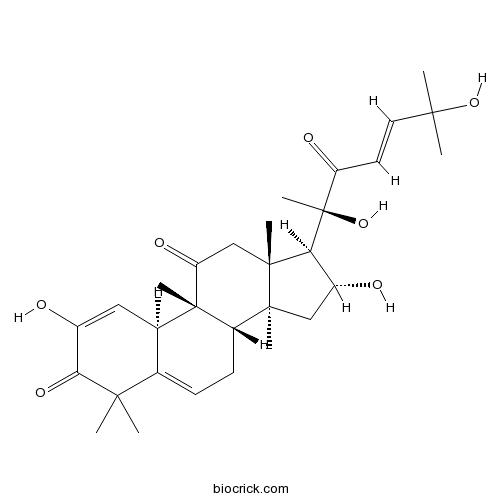
3D structure
| Cas No. | 2222-07-3 | SDF | Download SDF |
| PubChem ID | 5281321 | Appearance | White powder |
| Formula | C30H42O7 | M.Wt | 514.65 |
| Type of Compound | Isoprenoids | Storage | Desiccate at -20°C |
| Synonyms | Elatericin B, JSI 124 | ||
| Solubility | DMSO : ≥ 100 mg/mL (194.31 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (8S,9R,10R,13R,14S,16R,17R)-17-[(E,2R)-2,6-dihydroxy-6-methyl-3-oxohept-4-en-2-yl]-2,16-dihydroxy-4,4,9,13,14-pentamethyl-8,10,12,15,16,17-hexahydro-7H-cyclopenta[a]phenanthrene-3,11-dione | ||
| SMILES | CC1(C2=CCC3C4(CC(C(C4(CC(=O)C3(C2C=C(C1=O)O)C)C)C(C)(C(=O)C=CC(C)(C)O)O)O)C)C | ||
| Standard InChIKey | NISPVUDLMHQFRQ-MKIKIEMVSA-N | ||
| Standard InChI | InChI=1S/C30H42O7/c1-25(2,36)12-11-21(33)30(8,37)23-19(32)14-27(5)20-10-9-16-17(13-18(31)24(35)26(16,3)4)29(20,7)22(34)15-28(23,27)6/h9,11-13,17,19-20,23,31-32,36-37H,10,14-15H2,1-8H3/b12-11+/t17-,19-,20+,23+,27+,28-,29+,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of STAT3/JAK2 signaling. Inhibits the activation of STAT3 and JAK2 and displays no activity on Src, Akt, ERK and JNK. Suppresses phosphotyrosine levels of STAT3, inhibits STAT3 DNA binding and STAT3-mediated gene expression. Induces apoptosis in cell lines expressing constitutively active tyrosine-phosphorylated STAT3. |

Cucurbitacin I Dilution Calculator

Cucurbitacin I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9431 mL | 9.7153 mL | 19.4307 mL | 38.8614 mL | 48.5767 mL |
| 5 mM | 0.3886 mL | 1.9431 mL | 3.8861 mL | 7.7723 mL | 9.7153 mL |
| 10 mM | 0.1943 mL | 0.9715 mL | 1.9431 mL | 3.8861 mL | 4.8577 mL |
| 50 mM | 0.0389 mL | 0.1943 mL | 0.3886 mL | 0.7772 mL | 0.9715 mL |
| 100 mM | 0.0194 mL | 0.0972 mL | 0.1943 mL | 0.3886 mL | 0.4858 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cucurbitacin I is a selective inhibitor of JAK2/STAT3 signaling pathway with an IC50 value of 500 nM in A549 (a human lung adenocarcinoma cell line). It suppressed phosphotyrosine levels of STAT3, restrained STAT3 DNA binding and STAT3-mediated gene expression but had no effects on the activation of Src, Akt, ERK and JNK [1].
JAK/STAT3 signaling is well known for its vital role in the regulation of tumor cell proliferation, survival, invasion and immunosuppression. It promotes the development of various types of cancer in different manners [2].
Cucurbitacin I is often used to investigate the role of STAT3 in tumor development. It can induce apoptosis and block cell cycle progression of various cancer cells. In addition, Cucurbitacin I can decrease cell viability through inhibiting cell migration and invasion and enhancing chemosensitivity in the colon cancer cell line COLO205 [3].
Recent research has showed that it also has anti-angiogenic effects in human breast cancer cells [4]. In vivo, matrigel plug assay showed dramatic decrease in vascularization and hemoglobin content in the plugs from Cucurbitacin-I-treated mice, compared with control mice [5]. Therefore, Cucurbitacin I has potent anticancer effect on a variety of cancer cell types.
However, exposing glioblastoma multiforme cells to Cucurbitacin I could up-regulate beclin1 and trigger a protective autophagy against the apoptosis. Deletion of beclin 1 or treatment with the autophagy inhibitor sensitized cancer cells to Cucurbitacin I-induced apoptosis [6]. Thus the role of Cucurbitacin I in the regulation of autophagy requires for further research.
References:
Blaskovich MA, Sun J, Cantor A et al. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice.Cancer Res. 2003 Mar 15;63(6):1270-9.
Yu H, Lee H, Herrmann A et al. Revisiting STAT3 signalling in cancer: new and unexpected biological functions.Nat Rev Cancer. 2014 Nov;14(11):736-46. doi: 10.1038/nrc3818.
Song J, Liu H, Li Z et al. Cucurbitacin I inhibits cell migration and invasion and enhances chemosensitivity in colon cancer. Oncol Rep. 2015 Apr;33(4):1867-71.
Qi J, Xia G, Huang CR et al. JSI-124 (Cucurbitacin I) inhibits tumor angiogenesis of human breast cancer through reduction of STAT3 phosphorylation. Am J Chin Med. 2015;43(2):337-47.
Kim HJ, Kim JK et al. Antiangiogenic effects of cucurbitacin-I. Arch Pharm Res. 2015 Feb;38(2):290-8.
Yuan G, Yan SF, Xue H et al. Cucurbitacin I induces protective autophagy in glioblastoma in vitro and in vivo. J Biol Chem. 2014 Apr 11;289(15):10607-19.
- Dehydroglaucine
Catalog No.:BCN2548
CAS No.:22212-26-6
- Dihydroseselin
Catalog No.:BCN8258
CAS No.:2221-66-1
- Naproxen
Catalog No.:BCC9091
CAS No.:22204-53-1
- Pyrantel Pamoate
Catalog No.:BCC4958
CAS No.:22204-24-6
- Lyn peptide inhibitor
Catalog No.:BCC5895
CAS No.:222018-18-0
- L-Canavanine sulfate
Catalog No.:BCC6746
CAS No.:2219-31-0
- Macrocarpal N
Catalog No.:BCN5811
CAS No.:221899-21-4
- Zotarolimus(ABT-578)
Catalog No.:BCC5481
CAS No.:221877-54-9
- Methylswertianin
Catalog No.:BCN8505
CAS No.:22172-17-4
- Conocarpan
Catalog No.:BCN5053
CAS No.:221666-27-9
- OBAA
Catalog No.:BCC6716
CAS No.:221632-26-4
- L-Menthol
Catalog No.:BCN4971
CAS No.:2216-51-5
- Thalrugosaminine
Catalog No.:BCN7745
CAS No.:22226-73-9
- Fern-7-en-19-one
Catalog No.:BCN6443
CAS No.:222294-61-3
- 7-Amino-3-methyl-3-cephem-4-carboxylic acid
Catalog No.:BCC8776
CAS No.:22252-43-3
- Ipratropium Bromide
Catalog No.:BCC3795
CAS No.:22254-24-6
- Methyl 6-hydroxyangolensate
Catalog No.:BCN5054
CAS No.:22255-07-8
- alpha-Amyrin palmitate
Catalog No.:BCN5055
CAS No.:22255-10-3
- Guaijaverin
Catalog No.:BCN5056
CAS No.:22255-13-6
- Trimethoxystilbene
Catalog No.:BCN6762
CAS No.:22255-22-7
- Lucidin 3-O-glucoside
Catalog No.:BCN8249
CAS No.:22255-29-4
- Loganic acid
Catalog No.:BCN5057
CAS No.:22255-40-9
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- Tempol
Catalog No.:BCC4862
CAS No.:2226-96-2
Cucurbitacin B synergistically enhances the apoptosis-inducing effect of arsenic trioxide by inhibiting STAT3 phosphorylation in lymphoma Ramos cells.[Pubmed:28278714]
Leuk Lymphoma. 2017 Oct;58(10):2439-2451.
Arsenic trioxide (ATO) is a classic apoptosis-inducing agent used to treat acute promyelocytic leukemia. However, the therapeutic effect of ATO is limited in lymphoma, which resists apoptosis possibly due to inappropriate activation of STAT3. Therefore, combination of ATO and STAT3 inhibitor may be a potential strategy to treat lymphoma. Dramatically, Cucurbitacin B (CuB), an effective component of the dichloromethane extraction from Trichosanthes kirilowii Maxim, synergistically eliminated the apoptosis resistance of Burkitt's lymphoma Ramos cells to ATO by inhibiting the phosphorylation of STAT3, followed in turn by downregulation of Bcl-2 and upregulation of Bax. Furthermore, CuB and ATO in combination have no pro-apoptotic effect on normal lymphatic cells, indicating the absence of toxicity to hematological cells. This synergistic effect was further confirmed in nude murine lymphoma model, which exhibited significant apoptosis induction and tumor growth inhibition. Collectively, CuB synergistically enhances the apoptosis-inducing effect of ATO by inhibiting STAT3 phosphorylation in Ramos cells.
Comprehensive assessment of Cucurbitacin E related hepatotoxicity and drug-drug interactions involving CYP3A and P-glycoprotein.[Pubmed:28257659]
Phytomedicine. 2017 Mar 15;26:1-10.
BACKGROUND: Cucurbitacin E (CuE), a tetracyclic triterpenoid isolated from Cucurbitaceae, possesses many pharmacological activities especially anti-cancer. PURPOSE: The aim of this investigation was to comprehensively assess CuE related hepatotoxicity and potential drug-drug interactions involving CYP3A and P-glycoprotein (P-gp). STUDY DESIGN AND METHODS: Four common cytotoxicity assays (MTS, SRB, NRU and apoptosis assays) were used to evaluate the hepatotoxicity of CuE in human hepatocellular carcinoma HepG2 cells. Human and rat liver microsomes incubation system, Caco-2 transport model and 3D organoids model were used to investigate the effects of CuE on CYP3A and P-gp in vitro. The oral pharmacokinetics of indinavir was employed to evaluate the effects of CuE on CYP3A and P-gp in vivo. RESULTS: CuE induced the HepG2 apoptosis and exhibited acute cytotoxicity in MTS, SRB, and NRU assays with IC50 value at 15.98microM, 0.31microM, and 1.11microM, respectively. Moreover, CuE not only presented mechanism-based inhibition on human CYP3A4, but also decreased the efflux ratio of digoxin (P-gp substrate) across Caco-2 cell monolayers in vitro. Furthermore, CuE significantly inhibited the transport of Rh123 into 3D organoids, which was caused by the inhibition on P-gp. In Sprague-Dawley rat studies in vivo, acute administration of CuE significantly increased the maximum serum concentration (Cmax) and area under the concentration-time curve (AUC) of indinavir. In contrast, CuE treatment for three consecutive days significantly decreased indinavir Cmax and AUC in rats. CONCLUSION: These studies demonstrated that CuE has strong hepatotoxicity, and CuE presents potent inhibition on both CYP3A and P-gp activities in vitro. In animal in vivo studies, CuE induces CYP3A and P-gp after a long-term treatment but inhibits the activities of CYP3A and P-gp after an acute dosing. Therefore, CuE as a dual functional regulator of both CYP3A and P-gp may cause complex drug-drug interactions.
Cucurbitacin IIa exerts antidepressant-like effects on mice exposed to chronic unpredictable mild stress.[Pubmed:28240721]
Neuroreport. 2017 Mar 22;28(5):259-267.
Cucurbitacin IIa (CuIIa) is the major active component of the Helmseya amabilis root and is known to have antiviral and anti-inflammatory effects. In this study, we examined the antidepressant-like effects of CuIIa in a mouse model of chronic unpredictable mild stress (CUMS) and investigated the possible underlying mechanisms. To evaluate the antidepressant-like effects of CuIIa on depression-like behaviors, mice were subjected to the open-field test, the elevated plus-maze test, the forced-swimming test, and the tail-suspension test. We found that CuIIa treatment reversed the CUMS-induced behavioral abnormalities. Western blot analyses showed that CUMS significantly decreased brain-derived neurotrophic factor (BDNF) levels, cAMP-response element binding protein (CREB), and calcium/calmodulin-dependent protein kinase II (CaMKII) phosphorylation, and N-methyl-D-aspartate receptor subtype GluN2B and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor GluA1 expression in the amygdala; in addition, the expression of gamma-aminobutyric acid receptor A subunit alpha2 was upregulated in CUMS mice. These CUMS-induced changes were all normalized by CuIIa treatment and administration of the BDNF antagonist ANA-12 can block the antidepressant effect of CuIIa. Our findings suggest that the antidepressant-like effects of CuIIa may be exerted by regulation of the CaMKIIalpha-CREB-BDNF pathway and the balance between excitatory and inhibitory synaptic transmission in the amygdala.
Protective Effects of Cucurbitacin B on Acute Lung Injury Induced by Sepsis in Rats.[Pubmed:28315572]
Med Sci Monit. 2017 Mar 18;23:1355-1362.
BACKGROUND The aim of this study was to investigate the protective effects of cucurbitacin B (CuB) on sepsis-induced acute lung injury (ALI) in rats. MATERIAL AND METHODS An ALI model was made by cecal ligation and puncture (CLP) in SD rats. Rats were randomly divided into 5 groups (n=15 per group): animals undergoing a sham CLP (sham group); animals undergoing CLP (CLP control group); animals undergoing CLP and treated with CuB at 1 mg/kg of body weight (bw) (low-dose CuB [L-CuB] group), animals undergoing CuB at 2 mg/kg of bw (mid-dose CuB [M-CuB] group); and animals undergoing CuB at 5 mg/kg of bw (high-dose CuB [H-CuB] group). Samples of blood and lung tissue were harvested at different time points (6, 12, and 24 hour post-CLP surgery) for the detection of indicators which represented ALI. Five rats were respectively sacrificed at each time point. Pathological changes of lung tissue were observed by H&E staining. Another 50 rats were distributed into the same five groups to record the 72 hour survival rates. RESULTS Treatment with CuB significantly increased the blood gas PaO2 levels and decreased lung wet/dry (W/D) ratio (p<0.05). It significantly reduced protein concentration, accumulation of the inflammatory cells, and levels of tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6), (p<0.05), in the bronchoalveolar lavage fluid (BALF). Pulmonary pathological damage and survival rates at 72 hours were found to be effectively improved by CuB. In addition, CuB performed its pulmonary protection effects in a dose-depended manner. CONCLUSIONS CuB can effectively improve the pulmonary gas exchange function, reduce pulmonary edema, and inhibit the inflammatory response in the lung, revealing that CuB may serve as a potential therapeutic strategy for sepsis-induced ALI.
Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection.[Pubmed:17611279]
J Neurosci. 2007 Jul 4;27(27):7268-74.
Estradiol is protective in experimental cerebral ischemia, but the precise mechanisms remain unknown. Signal transducer and activator of transcription-3 (STAT3) is a transcription factor that is activated by estrogen, translocates to the nucleus, and induces the transcription of neuroprotective genes, such as bcl-2. We determined whether estradiol increases STAT3 activation in female rat brain after focal cerebral ischemia and whether STAT3 activation contributes to estradiol-mediated neuroprotection against ischemic brain injury. Ovariectomized (OVX) female rats with and without estradiol replacement were subjected to 2 h of middle cerebral artery occlusion (MCAO), and phosphorylated STAT3 (P-STAT3) and total STAT3 (T-STAT3) were quantified by Western blot analysis at 3 and 22 h of reperfusion. STAT3 activation was colocalized with neuronal and survival markers microtubule-associated protein 2 (MAP2) and Bcl-2 using immunohistochemistry. Infarct size was measured at 22 h after MCAO in estradiol-treated OVX animals in the presence and absence of STAT3 inhibitor Cucurbitacin I (JSI-124) using 2,3,5-triphenyltetrazolium chloride staining. Estradiol increased P-STAT3 in the ischemic cortex cytosolic fraction at 3 h after MCAO without affecting T-STAT3. This was associated with increased P-STAT3 in the nuclear fraction, which remained elevated at 22 h after MCAO. The nuclear P-STAT3 colocalized with MAP2 and Bcl-2 within the peri-infarct zone. The P-STAT3 inhibitor JSI-124 abolished the protective effect of estradiol without affecting infarct size in untreated OVX rats. We conclude that estradiol increases STAT3 phosphorylation in neurons after MCAO and that STAT3 activation plays an important role in estradiol-mediated neuroprotection.
Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway.[Pubmed:16230418]
Cancer Res. 2005 Oct 15;65(20):9525-35.
Abnormal dendritic cell differentiation and accumulation of immunosuppressive myeloid cells in cancer is one of the major factors of tumor nonresponsiveness. We have previously shown that hyperactivation of the Janus-activated kinase 2/signal transducers and activators of transcription 3 (JAK2/STAT3) induced by tumor-derived factors (TDF) is responsible for abnormal dendritic cell differentiation. Here, using a novel selective inhibitor of JAK2/STAT3 JSI-124, we investigated the possibility of pharmacologic regulation of dendritic cell differentiation in cancer. Our experiments in vitro have shown that JSI-124 overcomes the differentiation block induced by TDF and promotes the differentiation of mature dendritic cells and macrophages. JSI-124 significantly reduced the presence of immature myeloid cells in vivo and promoted accumulation of mature dendritic cells. In addition to a direct antitumor effect in several animal models, JSI-124 significantly enhanced the effect of cancer immunotherapy. This indicates that pharmacologic inhibition of the JAK2/STAT3 pathway can be an important new therapeutic strategy to enhance antitumor activity of cancer immunotherapy.
Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice.[Pubmed:12649187]
Cancer Res. 2003 Mar 15;63(6):1270-9.
Constitutively activated, tyrosine-phosphorylated signal transducer and activator of transcription (STAT) 3 plays a pivotal role in human tumor malignancy. To discover disrupters of aberrant STAT3 signaling pathways as novel anticancer drugs, we developed a phosphotyrosine STAT3 cytoblot. Using this high throughput 96-well plate assay, we identified JSI-124 (Cucurbitacin I) from the National Cancer Institute Diversity Set. JSI-124 suppressed the levels of phosphotyrosine STAT3 in v-Src-transformed NIH 3T3 cells and human cancer cells potently (IC(50) value of 500 nM in the human lung adenocarcinoma A549) and rapidly (complete inhibition within 1-2 h). The suppression of phosphotyrosine STAT3 levels resulted in the inhibition of STAT3 DNA binding and STAT3-mediated but not serum response element-mediated gene transcription. JSI-124 also decreased the levels of tyrosine-phosphorylated Janus kinase (JAK) but not those of Src. JSI-124 was highly selective for JAK/STAT3 and did not inhibit other oncogenic and tumor survival pathways such as those mediated by Akt, extracellular signal-regulated kinase 1/2, or c-Jun NH(2)-terminal kinase. Finally, JSI-124 (1 mg/kg/day) potently inhibited the growth in nude mice of A549 tumors, v-Src-transformed NIH 3T3 tumors, and the human breast carcinoma MDA-MB-468, all of which express high levels of constitutively activated STAT3, but it did not affect the growth of oncogenic Ras-transformed NIH 3T3 tumors that are STAT3 independent or of the human lung adenocarcinoma Calu-1, which has barely detectable levels of phosphotyrosine STAT3. JSI-124 also inhibited tumor growth and significantly increased survival of immunologically competent mice bearing murine melanoma with constitutively activated STAT3. These results give strong support for pharmacologically targeting the JAK/STAT3 signaling pathway for anticancer drug discovery.


