DIPPA hydrochlorideCAS# 155512-52-0 |
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 155512-52-0 | SDF | Download SDF |
| PubChem ID | 45073425 | Appearance | Powder |
| Formula | C22H24Cl3N3OS | M.Wt | 484.87 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 30 mM in ethanol and to 50 mM in DMSO | ||
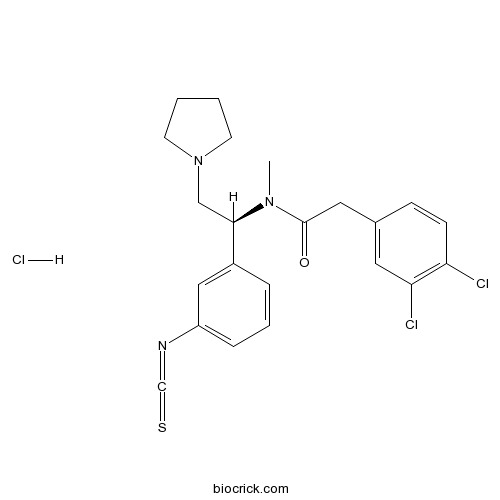
| Chemical Name | 2-(3,4-dichlorophenyl)-N-[(1S)-1-(3-isothiocyanatophenyl)-2-pyrrolidin-1-ylethyl]-N-methylacetamide;hydrochloride | ||
| SMILES | CN(C(CN1CCCC1)C2=CC(=CC=C2)N=C=S)C(=O)CC3=CC(=C(C=C3)Cl)Cl.Cl | ||
| Standard InChIKey | BNWYENYHNOESCX-ZMBIFBSDSA-N | ||
| Standard InChI | InChI=1S/C22H23Cl2N3OS.ClH/c1-26(22(28)12-16-7-8-19(23)20(24)11-16)21(14-27-9-2-3-10-27)17-5-4-6-18(13-17)25-15-29;/h4-8,11,13,21H,2-3,9-10,12,14H2,1H3;1H/t21-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | An irreversible and selective antagonist at the κ receptor, with persistent effect in vivo. |

DIPPA hydrochloride Dilution Calculator

DIPPA hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0624 mL | 10.312 mL | 20.6241 mL | 41.2482 mL | 51.5602 mL |
| 5 mM | 0.4125 mL | 2.0624 mL | 4.1248 mL | 8.2496 mL | 10.312 mL |
| 10 mM | 0.2062 mL | 1.0312 mL | 2.0624 mL | 4.1248 mL | 5.156 mL |
| 50 mM | 0.0412 mL | 0.2062 mL | 0.4125 mL | 0.825 mL | 1.0312 mL |
| 100 mM | 0.0206 mL | 0.1031 mL | 0.2062 mL | 0.4125 mL | 0.5156 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,9-Caryolanediol 9-acetate
Catalog No.:BCN1698
CAS No.:155488-34-9
- 3,6-Caryolanediol
Catalog No.:BCN1697
CAS No.:155485-76-0
- JWH 015
Catalog No.:BCC5744
CAS No.:155471-08-2
- 4,5-Dihydroblumenol A
Catalog No.:BCN1696
CAS No.:155418-97-6
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid methyl ester
Catalog No.:BCC8671
CAS No.:155405-80-4
- Ethyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN3302
CAS No.:155401-23-3
- Btk inhibitor 1 R enantiomer hydrochloride
Catalog No.:BCC5126
CAS No.:1553977-42-6
- p-Menthane-1,3,8-triol
Catalog No.:BCN1695
CAS No.:155348-06-4
- Fluconazole hydrate
Catalog No.:BCC4235
CAS No.:155347-36-7
- (S)-Sulforaphane
Catalog No.:BCC8097
CAS No.:155320-20-0
- Alisol E 23-acetate
Catalog No.:BCN3459
CAS No.:155301-58-9
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Alisol F
Catalog No.:BCN3360
CAS No.:155521-45-2
- Alisol G
Catalog No.:BCN3461
CAS No.:155521-46-3
- 3a-Epiburchellin
Catalog No.:BCN7015
CAS No.:155551-61-4
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- VUF 10166
Catalog No.:BCC5060
CAS No.:155584-74-0
- Dehydroaglaiastatin
Catalog No.:BCN1699
CAS No.:155595-93-0
- PG-9 maleate
Catalog No.:BCC6779
CAS No.:155649-00-6
- Notoginsenoside Ft1
Catalog No.:BCN6434
CAS No.:155683-00-4
- Simonsinol
Catalog No.:BCN1700
CAS No.:155709-40-3
- Isomagnolone
Catalog No.:BCN1701
CAS No.:155709-41-4
- Mecarbinate
Catalog No.:BCC4919
CAS No.:15574-49-9
- Pizotifen
Catalog No.:BCC4215
CAS No.:15574-96-6
kappa Opioid receptor selective affinity labels: electrophilic benzeneacetamides as kappa-selective opioid antagonists.[Pubmed:7799399]
J Med Chem. 1994 Dec 23;37(26):4490-8.
2-(3,4-Dichlorophenyl)-N-methyl-N-[1-(3- or 4-substituted phenyl)-2-(1-pyrrolidinyl)ethyl]-acetamides 3-6 were synthesized as kappa-selective affinity labels and evaluated for opioid activity. In smooth muscle preparations, the non-electrophilic parent compound (+)-S-2 and the affinity labels 3-6 behaved as kappa agonists in that they were potently antagonized by norbinaltorphimine (norBNI). In addition to the high binding affinity and selectivity of the 3-isothiocyanate 3 (DIPPA) to kappa opioid receptors, wash studies have suggested that this involves covalent binding. In the mouse tail-flick assay, the 3- and 4-substituted isomers (3 and 5, respectively) produced long-lasting antagonism of the antinociceptive effect of the kappa opioid agonist, (+/-)-trans-2-(3,4-dichlorophenyl)-N-methyl-N-[2-(1-pyrrolidinyl) cyclohexyl]acetamide ((+/-)-U50,488). In contrast, the non-electrophilic parent compound (+)-S-2 and the fumaramate derivative 4 were devoid of antagonist activity in the tail-flick assay. At substantially different doses, DIPPA (3) and the 4-isothiocyanate 5 also produced antinociception in the mouse abdominal stretch assay. In addition, DIPPA and the 3-fumaramate methyl ester 4 had improved in vivo kappa-selectivities compared to the unsubstituted parent compound (+)-S-2 and the para-substituted derivative 5. The improved kappa-selectivities of 3 and 4 and the different agonist and antagonist potencies of 3 and 5 may be explained respectively by the existence of multiple kappa agonist binding sites and distinct agonist and antagonist binding sites. In view of the antagonist selectivity and the apparent irreversible binding of DIPPA to kappa receptors, it may serve as a useful pharmacologic or biochemical tool to investigate kappa opioid receptors.


