DaminozideKDM2A inhibitor CAS# 1596-84-5 |
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- Tasquinimod
Catalog No.:BCC1987
CAS No.:254964-60-8
- CHAPS
Catalog No.:BCC1476
CAS No.:75621-03-3
Quality Control & MSDS
Number of papers citing our products

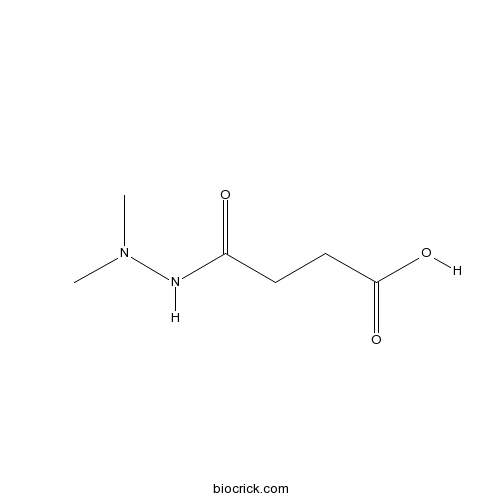
Chemical structure
3D structure
| Cas No. | 1596-84-5 | SDF | Download SDF |
| PubChem ID | 15331 | Appearance | Powder |
| Formula | C6H12N2O3 | M.Wt | 160.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 106.67 mg/mL (665.98 mM; Need warming) | ||
| Chemical Name | 4-(2,2-dimethylhydrazinyl)-4-oxobutanoic acid | ||
| SMILES | CN(C)NC(=O)CCC(=O)O | ||
| Standard InChIKey | NOQGZXFMHARMLW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H12N2O3/c1-8(2)7-5(9)3-4-6(10)11/h3-4H2,1-2H3,(H,7,9)(H,10,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Histone demethylase KDM2/7 subfamily inhibitor (IC50 values are 0.55, 1.5 and 2.1 μM for PHF8, KDM2A and KDM7A respectively). Exhibits ≥ 60-fold selectivity for KDM2/7 demethylases over other histone demethylases and 2-oxoglurate oxygenases (IC50 > 100 μM). |

Daminozide Dilution Calculator

Daminozide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.2434 mL | 31.2168 mL | 62.4337 mL | 124.8673 mL | 156.0842 mL |
| 5 mM | 1.2487 mL | 6.2434 mL | 12.4867 mL | 24.9735 mL | 31.2168 mL |
| 10 mM | 0.6243 mL | 3.1217 mL | 6.2434 mL | 12.4867 mL | 15.6084 mL |
| 50 mM | 0.1249 mL | 0.6243 mL | 1.2487 mL | 2.4973 mL | 3.1217 mL |
| 100 mM | 0.0624 mL | 0.3122 mL | 0.6243 mL | 1.2487 mL | 1.5608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Daminozide, a plant growth regulator, selectively inhibits the KDM2A with IC50 value of 1.5 μM, PHF8 with IC50 value of 0.55 μM, KDM7A with IC50 value of 2 μM.[1]
FBXL11/KDM2A is a histone H3 lysine 36 demethylase enzyme which enzymatic activity relies on a conserved JmjC domain in the N-terminus of the protein that coordinates iron and alphaketoglutarate to catalyze demethylation via a hydroxylation based mechanism.[2] The ZF-CxxC DNA binding domain within FBXL11/KDM2A has the capacity to interact with non-methylated DNA and can target to CpG island regions of the genome where it specifically removes histone H3 lysine 36 methylation.[3] This mechanism acts to create a chromatin environment at CpG islands that highlights these regulatory elements and differentiates them from non-regulatory regions in large complex mammalian genomes. In a study in mouse hepatocytes, this gene was shown to regulate hepatic gluconeogenesis.[4]
Histone Nε-methyl lysine demethylases KDM2/7 have been identified as potential targets for cancer therapies. Lung cancer is the leading cause of cancer deaths in the United States and worldwide. Non–small cell lung cancer (NSCLC)accounts for about 85% of all lung cancer cases, and its molecular etiology is heterogeneous[5]. Klaus W. et al [6] found that KDM2A overexpression in NSCLC cells increased cell proliferation and invasiveness.And KDM2A knockdown abrogated tumor growth and invasive abilities of NSCLC cells in mouse xenograft models, suggesting that KDM2A may be a promising therapeutic target in NSCLC.Daminozide, as a KDM2A selective inhibitor, was once widely used as a plant growth retardant but now will be a potent approach to targeted therapies for NSCLC.[7]
References:
1.Nathan R. Rose. et al. Plant Growth Regulator Daminozide Is a Selective Inhibitor of Human KDM2/7 Histone Demethylases. Journal of Medicinal Chemistry. J. Med. Chem. 2012, 55: 6639−6643.
2.Tsukada Y. et al. "Histone demethylation by a family of JmjC domain-containing proteins". Nature 2006, 439 (7078): 811–6.
3.Blackledge NP. et al. "CpG islands recruit a histone H3 lysine 36 demethylase". Molecular Cell 2010, 38 (2): 179–90..
4.Pan D, Mao C. et al. "The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis". PLOS Genetics,2012, 8 (6): e1002761.
5.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380.
6.Klaus W.et al. KDM2A promotes lung tumorigenesis by epigenetically enhancing ERK1/2 signaling. J Clin Invest. 2013,123(12):5231-5246.
7.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181.
- GR 55562 dihydrochloride
Catalog No.:BCC6913
CAS No.:159533-25-2
- Fluconazole mesylate
Catalog No.:BCC4236
CAS No.:159532-41-9
- Enfuvirtide
Catalog No.:BCC5641
CAS No.:159519-65-0
- N-Acetyl-O-phosphono-Tyr-Glu-Glu-Ile-Glu
Catalog No.:BCC5853
CAS No.:159439-02-8
- Everolimus (RAD001)
Catalog No.:BCC3594
CAS No.:159351-69-6
- MPDC
Catalog No.:BCC6873
CAS No.:159262-32-5
- PNU 22394 hydrochloride
Catalog No.:BCC7285
CAS No.:15923-42-9
- Isokaempferide
Catalog No.:BCN3790
CAS No.:1592-70-7
- L-NIO dihydrochloride
Catalog No.:BCC6689
CAS No.:159190-44-0
- MM 77 dihydrochloride
Catalog No.:BCC6854
CAS No.:159187-70-9
- L-755,507
Catalog No.:BCC7282
CAS No.:159182-43-1
- CARIPORIDE
Catalog No.:BCC6432
CAS No.:159138-80-4
- 3,4-Secotirucalla-4(28,7,24-triene-3),26-dioic acid
Catalog No.:BCN1549
CAS No.:159623-48-0
- Saropyrone
Catalog No.:BCN7692
CAS No.:159650-12-1
- Wikstrol A
Catalog No.:BCN7938
CAS No.:159736-35-3
- ISRIB (trans-isomer)
Catalog No.:BCC5340
CAS No.:1597403-47-8
- Ibutamoren Mesylate
Catalog No.:BCC1638
CAS No.:159752-10-0
- Fmoc-Lys(Ac)-OH
Catalog No.:BCC3514
CAS No.:159766-56-0
- Ulipristal
Catalog No.:BCC4944
CAS No.:159811-51-5
- Sikokianin C
Catalog No.:BCN6827
CAS No.:159813-69-1
- 4'-O-Methylirenolone
Catalog No.:BCN7174
CAS No.:159853-36-8
- Mc-Val-Cit-PABC-PNP
Catalog No.:BCC4028
CAS No.:159857-81-5
- 3,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6491
CAS No.:159934-13-1
- NIBR189
Catalog No.:BCC8056
CAS No.:1599432-08-2
Quartz crystal microbalance for the determination of daminozide using molecularly imprinted polymers as recognition element.[Pubmed:16621501]
Biosens Bioelectron. 2007 Jan 15;22(6):1087-91.
As the Daminozide (DM) and its metabolite have been identified to be potentially carcinogenic, rapid detection method for them is necessary for food safety. A type of piezoelectric crystal sensor has been prepared by using a molecularly imprinted polymer (MIP) as recognition element. The molecularly imprinted polymer was prepared by hot-induced precipitation polymerization, and then the polymer particles were fixed on the surface of the electrode. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) were employed to evaluate the obtained imprinted polymer particles and the MIP sensitive film coated on the electrode. The results showed that a typical time-response curve of the MIP-coated crystal to the DM solution had been given, frequency shifts versus logarithm changes of DM showed good linear correlation within the concentration range of 1.0x10(-9) to 10(-6) mg/mL (y=11.38 lg x+115.45, r=0.9872) and 1.0x10(-6) to 10(-1) mg/mL (y=25.22lgx+209.44, r=0.9938), respectively. The detection limit was 5.0x10(-8) mg/mL (S/N=3), which is lower than that of conventional methods. Further, computer simulation technology was employed to investigate the interaction between methacrylic acid and DM for elucidating the recognition mechanism. The influencing factor pH has also been investigated. The injection experiments of DM structurally related compounds indicated that the obtained sensor has high sensitivity, excellent selectivity, low cost, good reproducibility, and reusable property by combining with piezoelectric crystal and molecularly imprinted polymer.
Influence of gibberellin and daminozide on the expression of terpene synthases and on monoterpenes in common sage (Salvia officinalis).[Pubmed:20163890]
J Plant Physiol. 2010 Jul 1;167(10):779-86.
Common sage (Salvia officinalis L., Lamiaceae) is one of the most important medicinal and aromatic plants, with antioxidant, antimicrobial, spasmolytic, astringent, antihidrotic and specific sensorial properties. The essential oil of the plant, composed mainly of the monoterpenes 1,8-cineole, alpha-thujone, beta-thujone and camphor, is responsible for some of these effects. Gibberellins regulate diverse physiological processes in plants, such as seed germination, shoot elongation and cell division. In this study, we analyzed the effect of exogenously applied plant growth regulators, namely gibberellic acid (GA(3)) and Daminozide, on leaf morphology and essential oil formation of two leaf stages during the period of leaf expansion. Essential oil content increased with increasing levels of gibberellins and decreased when gibberellin biosynthesis was blocked with Daminozide. With increasing levels of gibberellins, 1,8-cineole and camphor contents increased. Daminozide blocked the accumulation of alpha- and beta-thujone. GA(3) at the highest level applied also led to a significant decrease of alpha- and beta-thujone. Monoterpene synthases are a class of enzymes responsible for the first step in monoterpene biosynthesis, competing for the same substrate geranylpyrophosphate. The levels of gene expression of the three most important monoterpene synthases in sage were investigated, 1,8-cineole synthase leading directly to 1,8-cineole, (+)-sabinene synthase responsible for the first step in the formation of alpha- and beta-thujone, and (+)-bornyl diphosphate synthase, the first step in camphor biosynthesis. The foliar application of GA(3) increased, while Daminozide significantly decreased gene expression of the monoterpene synthases. The amounts of two of the end products, 1,8-cineole and camphor, were directly correlated with the levels of gene expression of the respective monoterpene synthases, indicating transcriptional control, while the formation of alpha- and beta-thujone was not transcriptionally regulated.
Plant growth regulator daminozide is a selective inhibitor of human KDM2/7 histone demethylases.[Pubmed:22724510]
J Med Chem. 2012 Jul 26;55(14):6639-43.
The JmjC oxygenases catalyze the N-demethylation of N(epsilon)-methyl lysine residues in histones and are current therapeutic targets. A set of human 2-oxoglutarate analogues were screened using a unified assay platform for JmjC demethylases and related oxygenases. Results led to the finding that Daminozide (N-(dimethylamino)succinamic acid, 160 Da), a plant growth regulator, selectively inhibits the KDM2/7 JmjC subfamily. Kinetic and crystallographic studies reveal that Daminozide chelates the active site metal via its hydrazide carbonyl and dimethylamino groups.
Opposing effects of external gibberellin and Daminozide on Stevia growth and metabolites.[Pubmed:25342260]
Appl Biochem Biotechnol. 2015 Jan;175(2):780-91.
Steviol glycosides (SVglys) and gibberellins are originated from the shared biosynthesis pathway in Stevia (Stevia rebaudiana Bertoni). In this research, two experiments were conducted to study the opposing effects of external gibberellin (GA3) and Daminozide (a gibberellin inhibitor) on Stevia growth and metabolites. Results showed that GA3 significantly increased the stem length and stem dry weight in Stevia. Total soluble sugar content increased while the SVglys biosynthesis was decreased by external GA3 applying in Stevia leaves. In another experiment, the stem length was reduced by Daminozide spraying on Stevia shoots. The Daminozide did not affect the total SVglys content, while in 30 ppm concentration, significantly increased the soluble sugar production in Stevia leaves. Although the gibberellins biosynthesis pathway has previously invigorated in Stevia leaf, the Stevia response to external gibberellins implying on high precision regulation of gibberellins biosynthesis in Stevia and announces that Stevia is able to kept endogenous gibberellins in a low quantity away from SVglys production. Moreover, the assumption that the internal gibberellins were destroyed by Daminozide, lack of Daminozide effects on SVglys production suggests that gibberellins biosynthesis could not act as a competitive factor for SVglys production in Stevia leaves.


