Decanoyl-RVKR-CMKSubtilisin/Kex2p-like proprotein convertase inhibitor CAS# 150113-99-8 |
- Naphthoquine phosphate
Catalog No.:BCC1784
CAS No.:173531-58-3
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 150113-99-8 | SDF | Download SDF |
| PubChem ID | 9962075 | Appearance | Powder |
| Formula | C34H66ClN11O5 | M.Wt | 744.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
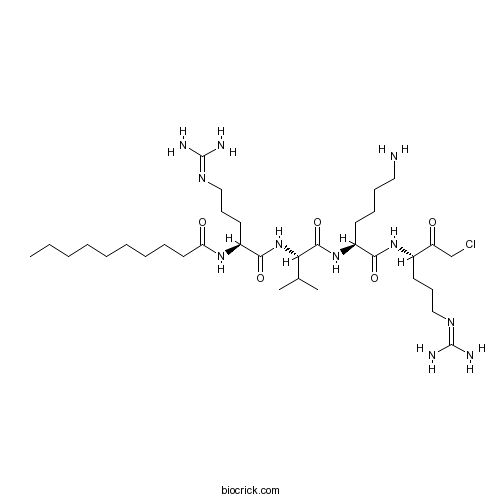
| Sequence | RVKR (Modifications: Arg-1 = Decanoyl-Arg, Arg-4 = chlororomethylketone) | ||
| Chemical Name | N-[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(3S)-1-chloro-6-(diaminomethylideneamino)-2-oxohexan-3-yl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]decanamide | ||
| SMILES | CCCCCCCCCC(=O)NC(CCCN=C(N)N)C(=O)NC(C(C)C)C(=O)NC(CCCCN)C(=O)NC(CCCN=C(N)N)C(=O)CCl | ||
| Standard InChIKey | NHBJTTGFHCHQHS-VZTVMPNDSA-N | ||
| Standard InChI | InChI=1S/C34H66ClN11O5/c1-4-5-6-7-8-9-10-18-28(48)43-25(17-14-21-42-34(39)40)31(50)46-29(23(2)3)32(51)45-26(15-11-12-19-36)30(49)44-24(27(47)22-35)16-13-20-41-33(37)38/h23-26,29H,4-22,36H2,1-3H3,(H,43,48)(H,44,49)(H,45,51)(H,46,50)(H4,37,38,41)(H4,39,40,42)/t24-,25-,26-,29-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Subtilisin/Kex2p-like proprotein convertase inhibitor; blocks activity of all seven convertases (PC1, PC2, PC4, PACE4, PC5, PC7 and furin). Abolishes proET-1 processing in endothelial cells; inhibits regulated secretion of the neuronal polypeptide VGF in PC12 cells. |

Decanoyl-RVKR-CMK Dilution Calculator

Decanoyl-RVKR-CMK Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Talabostat mesylate
Catalog No.:BCC5357
CAS No.:150080-09-4
- Methyl 2-ethoxybenzimidazole-7-carboxylate
Catalog No.:BCC9036
CAS No.:150058-27-8
- Cycloshizukaol A
Catalog No.:BCN6567
CAS No.:150033-85-5
- Phytol
Catalog No.:BCN1673
CAS No.:150-86-7
- Mequinol
Catalog No.:BCC4797
CAS No.:150-76-5
- m-Methoxyphenol
Catalog No.:BCN1669
CAS No.:150-19-6
- O-Acetylschisantherin L
Catalog No.:BCN3635
CAS No.:149998-51-6
- Gelomuloside B
Catalog No.:BCN6640
CAS No.:149998-39-0
- Gelomuloside A
Catalog No.:BCN6639
CAS No.:149998-38-9
- 5-O-Methyldalbergiphenol
Catalog No.:BCN8104
CAS No.:1499946-35-8
- Z-His-OH
Catalog No.:BCC2768
CAS No.:14997-58-1
- 9-Aminominocycline hydrochloride
Catalog No.:BCC8797
CAS No.:149934-21-4
- Qianhucoumarin A
Catalog No.:BCN3615
CAS No.:150135-35-6
- (S)-MCPG
Catalog No.:BCC6611
CAS No.:150145-89-4
- Strictosidinic acid
Catalog No.:BCN6965
CAS No.:150148-81-5
- 11,13-Dihydroivalin
Catalog No.:BCN4705
CAS No.:150150-61-1
- Crucigasterin 277
Catalog No.:BCN1777
CAS No.:150151-83-0
- Crucigasterin 275
Catalog No.:BCN1776
CAS No.:150151-84-1
- Crucigasterin 225
Catalog No.:BCN1786
CAS No.:150151-85-2
- BIM 23056
Catalog No.:BCC5824
CAS No.:150155-61-6
- Trigothysoid N
Catalog No.:BCN6881
CAS No.:1501943-08-3
- 12-Deoxo-12alpha-acetoxyelliptone
Catalog No.:BCN4803
CAS No.:150226-21-4
- Fmoc-2-Abz-OH
Catalog No.:BCC3204
CAS No.:150256-42-1
- Fmoc-p-amino-benzoic acid,Fmoc-4-Abz-OH
Catalog No.:BCC2622
CAS No.:15026-42-1
Mature pig oligodendrocytes rapidly process human recombinant pro-nerve growth factor and do not undergo cell death.[Pubmed:16805842]
J Neurochem. 2006 Jul;98(2):506-17.
The neurotrophin family with its first member, nerve growth factor (NGF), binds two classes of receptors, more specifically to Trk receptors and to a shared p75NTR receptor. It has been shown that proNGF rather than NGF is predominant in the mature central nervous system. A recent finding indicated that a furin-resistant proNGF preferentially binds to p75NTR, initiating a pro-apoptotic cascade even in the presence of TrkA. In this context, rodent oligodendrocytes were reported to undergo cell death when exposed to proNGF. We have investigated the effect of a non-mutated 32 kDa human recombinant proNGF (rhproNGF) on cultured pig oligodendrocytes which express TrkA, p75NTR and sortilin. Pig oligodendrocytes respond to rhproNGF (50 ng/mL) with an enhanced regeneration of their processes as already observed for NGF. Activity of mitogen-activated protein kinase (MAPK), which plays an important role in oligodendroglial process formation, was increased even when rhproNGF processing was inhibited by the furin inhibitor Decanoyl-RVKR-CMK. Similarly, a cleavage-resistant proNGF (R-1G) activated MAPK and promoted oligodendroglial process regeneration. High concentrations of rhproNGF (300 ng/mL) did not induce cell death. Sodium dodecyl sulfate - polyacrylamide gel electrophoresis and Western blotting revealed that oligodendrocytes process rhproNGF to NGF. NGF was detected in Western blots of oligodendroglial lysates already 10 min after rhproNGF exposure, followed by a release of NGF into the culture medium. Indirect evidence indicates that rhproNGF processing occurs via an endocytotic route.
Comparative study of the binding pockets of mammalian proprotein convertases and its implications for the design of specific small molecule inhibitors.[Pubmed:20151049]
Int J Biol Sci. 2010 Feb 3;6(1):89-95.
Proprotein convertases are enzymes that proteolytically cleave protein precursors in the secretory pathway to yield functional proteins. Seven mammalian subtilisin/Kex2p-like proprotein convertases have been identified: furin, PC1, PC2, PC4, PACE4, PC5 and PC7. The binding pockets of all seven proprotein convertases are evolutionarily conserved and highly similar. Among the seven proprotein convertases, the furin cleavage site motif has recently been characterized as a 20-residue motif that includes one core region P6-P2' inside the furin binding pocket. This study extended this information by examining the 3D structural environment of the furin binding pocket surrounding the core region P6-P2' of furin substrates. The physical properties of mutations in the binding pockets of the other six mammalian proprotein convertases were compared. The results suggest that: 1) mutations at two positions, Glu230 and Glu257, change the overall density of the negative charge of the binding pockets, and govern the substrate specificities of mammalian proprotein convertases; 2) two proprotein convertases (PC1 and PC2) may have reduced sensitivity for positively charged residues at substrate position P5 or P6, whereas the substrate specificities of three proprotein convertases (furin, PACE4, and PC5) are similar to each other. This finding led to a novel design of a short peptide pattern for small molecule inhibitors: [K/R]-X-V-X-K-R. Compared with the widely used small molecule dec-RVKR-cmk that inhibits all seven proprotein convertases, a finely-tuned derivative of the short peptide pattern [K/R]-X-V-X-K-R may have the potential to more effectively inhibit five of the proprotein convertases (furin, PC4, PACE4, PC5 and PC7) compared to the remaining two (PC1 and PC2). The results not only provide insights into the molecular evolution of enzyme function in the proprotein convertase family, but will also aid the study of the functional redundancy of proprotein convertases and the development of therapeutic applications.
A prohormone convertase cleavage site within a predicted alpha-helix mediates sorting of the neuronal and endocrine polypeptide VGF into the regulated secretory pathway.[Pubmed:16221685]
J Biol Chem. 2005 Dec 16;280(50):41595-608.
Distinct intracellular pathways are involved in regulated and constitutive protein secretion from neuronal and endocrine cells, yet the peptide signals and molecular mechanisms responsible for targeting and retention of soluble proteins in secretory granules are incompletely understood. By using confocal microscopy and subcellular fractionation, we examined trafficking of the neuronal and endocrine peptide precursor VGF that is stored in large dense core vesicles and undergoes regulated secretion. VGF cofractionated with secretory vesicle membranes but was not detected in detergent-resistant lipid rafts. Deletional analysis using epitope-tagged VGF suggested that the C-terminal 73-amino acid fragment of VGF, containing two predicted alpha-helical loops and four potential prohormone convertase (PC) cleavage sites, was necessary and sufficient with an N-terminal signal peptide-containing domain, for large dense core vesicle sorting and regulated secretion from PC12 and INS-1 cells. Further transfection analysis identified the sorting sequence as a compact C-terminal alpha-helix and embedded 564RRR566 PC cleavage site; mutation of the 564RRR566 PC site in VGF-(1-65): GFP:VGF-(545-617) blocked regulated secretion, whereas disruption of the alpha-helix had no effect. Mutation of the adjacent 567HFHH570 motif, a charged region that might enhance PC cleavage in acidic environments, also blocked regulated release. Finally, inhibition of PC cleavage in PC12 cells using the membrane-permeable synthetic peptide chloromethyl ketone (Decanoyl-RVKR-CMK) blocked regulated secretion of VGF. Our studies define a critical RRR-containing C-terminal domain that targets VGF into the regulated pathway in neuronal PC12 and endocrine INS-1 cells, providing additional support for the proposed role that PCs and their cleavage sites play in regulated peptide secretion.
Inhibition of convertase-related processing of proendothelin-1.[Pubmed:8587448]
J Cardiovasc Pharmacol. 1995;26 Suppl 3:S47-50.
The biologically inactive precursor proendothelin-1 (proET-1) is initially processed intracellularly to the intermediate big endothelin peptide (big ET-1) before its conversion to the endothelin-1 (ET-1) peptide by the endothelin-converting enzyme (ECE). We recently demonstrated that purified furin, a calcium-dependent serine endoprotease belonging to the family of mammalian convertases, cleaved proET-1 in vitro and hence produced the physiologically relevant big ET-1 peptide. Therefore, furin becomes a candidate proET-1-cleaving enzyme responsible for the initial biosynthetic processing steps of this precursor. In this study we examined the inhibitory properties of two convertase inhibitors, i.e., the decanoyl-Arg-Val-Lys-Arg-chloromethylketone (dec-RVKR-cmk) and the alpha 1-antitrypsin Portland (AT-PDX) on proET-1 processing. When purified furin or PACE4, another mammalian convertase, was incubated in the presence of dec-RVKR-cmk, proET-1 processing was completely abolished. In the presence of purified AT-PDX, furin-related cleavage of proET-1 was abolished but not PACE4 processing. In addition, incubation of endothelial cells with dec-RVKR-cmk inhibited production of ET-1 but did not significantly alter the levels of the prostanoid PGI2. These results indicate that convertase-related processing of proET-1 can be inhibited in vitro and in vivo, and that convertase-specific processing may be prevented with AT-PDX.


