Dp44mTiron chelator CAS# 152095-12-0 |
- Batimastat (BB-94)
Catalog No.:BCC1223
CAS No.:130370-60-4
- Batimastat sodium salt
Catalog No.:BCC2075
CAS No.:130464-84-5
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 152095-12-0 | SDF | Download SDF |
| PubChem ID | 10334137 | Appearance | Powder |
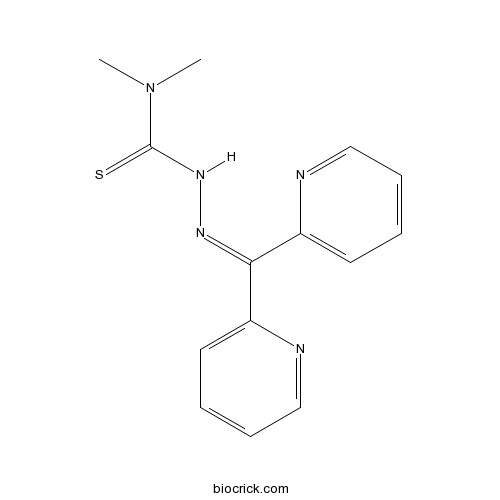
| Formula | C14H15N5S | M.Wt | 285.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (350.42 mM; Need ultrasonic) | ||
| Chemical Name | 3-(dipyridin-2-ylmethylideneamino)-1,1-dimethylthiourea | ||
| SMILES | CN(C)C(=S)NN=C(C1=CC=CC=N1)C2=CC=CC=N2 | ||
| Standard InChIKey | XOBIGRNRXCAMJQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H15N5S/c1-19(2)14(20)18-17-13(11-7-3-5-9-15-11)12-8-4-6-10-16-12/h3-10H,1-2H3,(H,18,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dp44mT Dilution Calculator

Dp44mT Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5042 mL | 17.5211 mL | 35.0422 mL | 70.0845 mL | 87.6056 mL |
| 5 mM | 0.7008 mL | 3.5042 mL | 7.0084 mL | 14.0169 mL | 17.5211 mL |
| 10 mM | 0.3504 mL | 1.7521 mL | 3.5042 mL | 7.0084 mL | 8.7606 mL |
| 50 mM | 0.0701 mL | 0.3504 mL | 0.7008 mL | 1.4017 mL | 1.7521 mL |
| 100 mM | 0.035 mL | 0.1752 mL | 0.3504 mL | 0.7008 mL | 0.8761 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dp44mT is an iron chelator that selectively inhibit topoisomerase IIα, with an GI50 value of ~100 nmol/L in the human breast cancer cell line MDA-MB-231 [1].
Topoisomerases play important roles in chromosome segregation, DNA synthesis, and transcription. Topoisomerase I and topoisomerase II are two major types of topoisomerases in eukaryotes. Human cells contain two isozymes of topoisomerase II, named topo IIa and topo IIβ [2].
In control experiments, the Nalm-6 leukemic top2α+/- cells expressed ~57% as much levels of top2α enzyme as the wild type cells. Treated with Dp44mT at 100 nmol/L, compared with the top2α+/+ cells, top2α+/- cells showed partial resistance to the cytotoxic effects of the drug.After the exposure to Dp44mT at 100 nmol/L, the top2α+/+ cells showed 31.7%, while the top2α+/- cells only showed 9.4% sub-G1 containing cells. In HeLa cells, transient siRNA-mediated knockdown of top2α resulted in a reduction of ~78% in the protein level for top2α. In top2α siRNA cells, a partial resistance to Dp44mT (0.1 and 0.3 µmol/L) was found at 72 hours, compared with the control siRNA-treated cells [1].
In vivo, 6 and 24 hours after the treatment with 0.1 and 1 µmol/L of Dp44mT, the treatment resulted in the covalent complex formation between DNA and top2α. No complex formation was found after the treatment when probed for top1 or top2β. Caspase inhibitor pretreatment did not rescue the formation of top2α complex, so the formed top2α-DNA complexes were not the secondary effect of apoptosis [1].
References:
[1]. Rao VA, Klein SR, Agama KK, et al. The iron chelator Dp44mT causes DNA damage and selective inhibition of topoisomerase IIα in breast cancer cells. Cancer research, 2009, 69(3): 948-957.
[2]. Tan KB, Dorman TE, Falls KM, et al. Topoisomerase IIα and topoisomerase IIβ genes: characterization and mapping to human chromosomes 17 and 3, respectively. Cancer Research, 1992, 52(1): 231-234.
- Epothilone B (EPO906, Patupilone)
Catalog No.:BCC1092
CAS No.:152044-54-7
- Epothilone A
Catalog No.:BCC1091
CAS No.:152044-53-6
- Sophoricoside
Catalog No.:BCN2294
CAS No.:152-95-4
- Vicine
Catalog No.:BCC8366
CAS No.:152-93-2
- Quinestrol
Catalog No.:BCC9132
CAS No.:152-43-2
- Verapamil HCl
Catalog No.:BCC4747
CAS No.:152-11-4
- Ac2-26
Catalog No.:BCC5825
CAS No.:151988-33-9
- 6- Methoxydihydrosanguinarine
Catalog No.:BCN8346
CAS No.:151890-26-5
- 2-Phenylmelatonin
Catalog No.:BCC6748
CAS No.:151889-03-1
- Kinsenoside
Catalog No.:BCN3858
CAS No.:151870-74-5
- cAMPS-Rp, triethylammonium salt
Catalog No.:BCC8082
CAS No.:151837-09-1
- AC 187
Catalog No.:BCC6018
CAS No.:151804-77-2
- 3,4-Dimethoxybenzamide
Catalog No.:BCN6565
CAS No.:1521-41-1
- 1,2-Epoxy-1-hydroxymethylpyrrolizidine
Catalog No.:BCN1557
CAS No.:15211-03-7
- Teuclatriol
Catalog No.:BCN1676
CAS No.:152110-17-3
- 2-chloro-11-cyclopentyl-5H-benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one
Catalog No.:BCC8568
CAS No.:1521197-43-2
- N,N'-Di-Boc-1H-pyrazole-1-carboxamidine
Catalog No.:BCC9065
CAS No.:152120-54-2
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- SB 203580
Catalog No.:BCC3663
CAS No.:152121-47-6
- PD 169316
Catalog No.:BCC3969
CAS No.:152121-53-4
- A 80426 mesylate
Catalog No.:BCC7336
CAS No.:152148-64-6
- Forrestin A
Catalog No.:BCN1677
CAS No.:152175-76-3
- Aphadilactone B
Catalog No.:BCN7646
CAS No.:1522004-68-7
- Aphadilactone C
Catalog No.:BCN7645
CAS No.:1522004-70-1
A mechanism for overcoming P-glycoprotein-mediated drug resistance: novel combination therapy that releases stored doxorubicin from lysosomes via lysosomal permeabilization using Dp44mT or DpC.[Pubmed:27906178]
Cell Death Dis. 2016 Dec 1;7(12):e2510.
The intracellular distribution of a drug can cause significant variability in both activity and selectivity. Herein, we investigate the mechanism by which the anti-cancer agents, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) and the clinically trialed, di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC), re-instate the efficacy of doxorubicin (DOX), in drug-resistant P-glycoprotein (Pgp)-expressing cells. Both Dp44mT and DpC potently target and kill Pgp-expressing tumors, while DOX effectively kills non-Pgp-expressing cancers. Thus, the combination of these agents should be considered as an effective rationalized therapy for potently treating advanced and resistant tumors that are often heterogeneous in terms of Pgp-expression. These studies demonstrate that both Dp44mT and DpC are transported into lysosomes via Pgp transport activity, where they induce lysosomal-membrane permeabilization to release DOX trapped within lysosomes. This novel strategy of loading lysosomes with DOX, followed by permeabilization with Dp44mT or DpC, results in the relocalization of stored DOX from its lysosomal 'safe house' to its nuclear targets, markedly enhancing cellular toxicity against resistant tumor cells. Notably, the combination of Dp44mT or DpC with DOX showed a very high level of synergism in multiple Pgp-expressing cell types, for example, cervical, breast and colorectal cancer cells. These studies revealed that the level of drug synergy was proportional to Pgp activity. Interestingly, synergism was ablated by inhibiting Pgp using the pharmacological inhibitor, Elacridar, or by inhibiting Pgp-expression using Pgp-silencing, demonstrating the importance of Pgp in the synergistic interaction. Furthermore, lysosomal-membrane stabilization inhibited the relocalization of DOX from lysosomes to the nucleus upon combination with Dp44mT or DpC, preventing synergism. This latter observation demonstrated the importance of lysosomal-membrane permeabilization to the synergistic interaction between these agents. The synergistic and potent anti-tumor efficacy observed between DOX and thiosemicarbazones represents a promising treatment combination for advanced cancers, which are heterogeneous and composed of non-Pgp- and Pgp-expressing tumor cells.
Response to the Letter to the Editor by D. Richardson: Analysis of the Interaction of Dp44mT with Human Serum Albumin and Calf Thymus DNA Using Molecular Docking and Spectroscopic Techniques.[Pubmed:27854349]
Int J Mol Sci. 2016 Nov 16;17(11). pii: ijms17111917.
This response refers to.
The iron chelator Dp44mT suppresses osteosarcoma's proliferation, invasion and migration: in vitro and in vivo.[Pubmed:28078009]
Am J Transl Res. 2016 Dec 15;8(12):5370-5385. eCollection 2016.
Di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT), the novel iron chelator, has been reported to inhibit the tumorigenesis and progression of various cancer cells, including neuroblastoma, neuroepithelioma and prostate cancer. However, whether Dp44mT has anticancer effects in osteosarcoma is still unknown. Here, we investigated the antitumor action of Dp44mT in osteosarcoma and its underlying mechanisms. A human osteosarcoma 143B cell line in vitro and 143B xenograft in nude mice in vivo were utilized, the anticancer effects of Dp44mT were examined through methods of MTT assay, transwell, wound healing assay, flow cytometry, western blot, immunohistochemistry and H&E staining. We showed that Dp44mT inhibits cell proliferation, invasion and migration in vitro. In addition, flow cytometry further illustrated that Dp44mT suppression of 143B cell proliferation, invasion and migration were partially due to induction of cell apoptosis, cell cycle arrest in S phase and ROS production. Also in vitro and in vivo, the expression levels of Bcl2, Bax, Caspase3, Caspase9, LC3-II, beta-catenin and its downstream targets such as C-myc and Cyclin D1 demonstrated that cell apoptosis and autophagy, as well as Wnt/beta-catenin pathway were involved in Dp44mT induced osteosarcoma suppression. The Dp44mT inhibition of osteosarcoma was further verified via animal models. The findings indicated that in vivo Dp44mT showed a significant reduction in the 143B xenograft tumor growth and metastasis. In conclusion, our data demonstrated that Dp44mT has effective anticancer capability in osteosarcoma and that may represent a promising treatment strategy for osteosarcoma.
Letter to the Editor: "Analysis of the Interaction of Dp44mT with Human Serum Albumin and Calf Thymus DNA Using Molecular Docking and Spectroscopic Techniques".[Pubmed:27854347]
Int J Mol Sci. 2016 Nov 16;17(11). pii: ijms17111916.
n/a.


