E 64dCysteine protease inhibitor CAS# 88321-09-9 |

- L 006235
Catalog No.:BCC2361
CAS No.:294623-49-7
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
- E-64-c
Catalog No.:BCC3588
CAS No.:76684-89-4
- Cysteine Protease inhibitor
Catalog No.:BCC5301
CAS No.:921625-62-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure
3D structure
| Cas No. | 88321-09-9 | SDF | Download SDF |
| PubChem ID | 65663 | Appearance | Powder |
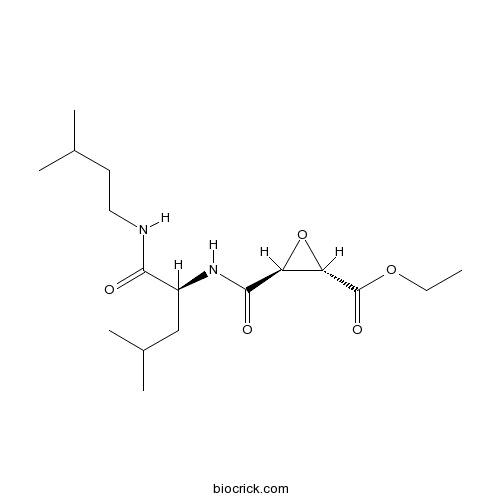
| Formula | C17H30N2O5 | M.Wt | 342.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | E64d; E64c ethyl ester | ||
| Solubility | Ethanol : ≥ 33.33 mg/mL (97.33 mM) DMSO : ≥ 23 mg/mL (67.17 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | ethyl (2S,3S)-3-[[(2S)-4-methyl-1-(3-methylbutylamino)-1-oxopentan-2-yl]carbamoyl]oxirane-2-carboxylate | ||
| SMILES | CCOC(=O)C1C(O1)C(=O)NC(CC(C)C)C(=O)NCCC(C)C | ||
| Standard InChIKey | SRVFFFJZQVENJC-IHRRRGAJSA-N | ||
| Standard InChI | InChI=1S/C17H30N2O5/c1-6-23-17(22)14-13(24-14)16(21)19-12(9-11(4)5)15(20)18-8-7-10(2)3/h10-14H,6-9H2,1-5H3,(H,18,20)(H,19,21)/t12-,13-,14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of cathepsins B and L; also thought to inhibit calpain. Inhibits lysosomal proteases and interferes with autolysosomal digestion when used in combination with pepstatin A. Lysosome and cell permeable. |

E 64d Dilution Calculator

E 64d Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9203 mL | 14.6015 mL | 29.203 mL | 58.4061 mL | 73.0076 mL |
| 5 mM | 0.5841 mL | 2.9203 mL | 5.8406 mL | 11.6812 mL | 14.6015 mL |
| 10 mM | 0.292 mL | 1.4602 mL | 2.9203 mL | 5.8406 mL | 7.3008 mL |
| 50 mM | 0.0584 mL | 0.292 mL | 0.5841 mL | 1.1681 mL | 1.4602 mL |
| 100 mM | 0.0292 mL | 0.146 mL | 0.292 mL | 0.5841 mL | 0.7301 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
E-64d inhibited hippocampal aberrant mossy fiber sprouting and seizure-induced up-regulation of ApoE and Clusterin in rats. A significant down-regulation of PRG-1, PRG-3, prg-5, cathepsin B amd ApoE and a up-regulation of nSMase and ANX7 were observed in hippocampus of E-64d-pretreated seizure rats.
Abstract
In order to detect autophage, E-64d, a membrane-permeable cysteine protease inhibitor, was applied in culture medium where root tips from Arabidopsis seedlings were incubated.
Abstract
E-64d exhibits protective effects against IRI-induced retinal apoptosis, since it inhibited IRI-induced up-regulation of m-calpain expression, the crease of m-calpain/calpastatin ratio and IRI-induced retinal damage in rats.
Abstract
E-64d is a membrane permeant inhibitor of calpain that enters intact cells and inhibits proteolysis once it’s incubated with platelets before lysis.
Abstract
CD-64L, CB-64D, CB-182 and CB-184 exhibited sigma 1 and sigma 2 Ki of 10.5 and 154 nM, 3063 and 16.5 nM, 27.3 and35.5 nM and 7436 and 13.4 nM respectively, where CB-64D and CB-184 displayed high sigma 2 receptor affinity and subtype selectivity.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
E-64d, a membrane permeant derivative of E-64c, a thiol protease inhibitor1, was tested for ability to inhibit calpain activity in intact platelets.
E-64c or E-64d also inhibited (lanes 3-8), demonstrating their effect on calpain. When the platelets were incubated with these inhibitors for I0 min and were then washed to remove extracellular inhibitor before lysis, neither E-64c nor leupeptin inhibited proteolysis, but E-64d did inhibit. E-64d was able to penetrate the platelet and was thus not removed by washing.E-64c failed to inhibit proteolysis in intact platelets, but E-64d, the permeant inhibitor, did inhibit intracellular proteolysis.E-64c and E-64d were each able to inhibit the protease activity in lysed platelets. This protease activity has been attributed to calpain by its absolute dependence on Ca 2+and by inhibition by known inhibitors of calpain. E-64d is able to enter the intact platelet: i) after washing to remove extracellular inhibitor, there was no protease activity detected after platelet lysis, and ii) activation of platelets preincubated with E-64d, but not E-64c, resulted in inhibition of proteolysis by calpain activated in intact platelets by A23187 plus calcium.
Reference:
1. M. Tamai, K. Matsumoto, S. Omura, I. Koyama, Y. Ozawa, K. Hanada J. Pharmacobio-Dyn., 9 (1986), pp. 672–677
2. E. B. McGowan, E. Becker, and T. C. Detwiler, INHIBITION OF CALPAIN IN INTACT PLATELETS BY THE THIOL PROTEASE INHIBITOR E-64d. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS , Vol. 158, No. 2, 1989
3. Carmen JC, Sinai AP. The Differential Effect of Toxoplasma Gondii Infection on the Stability of BCL2-Family Members Involves Multiple Activities. Front Microbiol. 2011 Jan 24;2:1.
- BI 78D3
Catalog No.:BCC8089
CAS No.:883065-90-5
- CCG 50014
Catalog No.:BCC4897
CAS No.:883050-24-6
- HC 067047
Catalog No.:BCC7861
CAS No.:883031-03-6
- Polygalasaponin F
Catalog No.:BCN2317
CAS No.:882664-74-6
- AMG-47A
Catalog No.:BCC6394
CAS No.:882663-88-9
- Netobimin
Catalog No.:BCC9100
CAS No.:88255-01-0
- R1530
Catalog No.:BCC1879
CAS No.:882531-87-5
- AI-3
Catalog No.:BCC8018
CAS No.:882288-28-0
- P005091
Catalog No.:BCC1287
CAS No.:882257-11-6
- H-Ile-OAll.TosOH
Catalog No.:BCC2963
CAS No.:88224-05-9
- H-Leu-OAll.TosOH
Catalog No.:BCC2969
CAS No.:88224-03-7
- Notopterol
Catalog No.:BCN5386
CAS No.:88206-46-6
- N-Benzyllinolenamide
Catalog No.:BCN6531
CAS No.:883715-18-2
- N-Benzyloleamide
Catalog No.:BCN1317
CAS No.:101762-87-2
- (9Z,12Z)-N-(3-Methoxybenzyl)octadeca-9,12-dienamide
Catalog No.:BCN1316
CAS No.:883715-22-8
- DPDPE
Catalog No.:BCC5758
CAS No.:88373-73-3
- Adrenorphin
Catalog No.:BCC1021
CAS No.:88377-68-8
- 8-Lavandulylkaempferol
Catalog No.:BCN3961
CAS No.:883859-83-4
- 8alpha-(2-Methylacryloyloxy)-1-O-methylhirsutinolide 13-O-acetate
Catalog No.:BCN7106
CAS No.:883872-71-7
- AZD2932
Catalog No.:BCC6388
CAS No.:883986-34-3
- Methyl syringate
Catalog No.:BCN4430
CAS No.:884-35-5
- L-(-)-α-Methyldopa hydrochloride
Catalog No.:BCC4083
CAS No.:884-39-9
- 6,7-Dihydroxycoumarin-4-Acetic Acid
Catalog No.:BCC9205
CAS No.:88404-14-2
- Buparvaquone
Catalog No.:BCC5437
CAS No.:88426-33-9
[Inhibition of calpain expression by E-64d in the rat retina subjected to ischemia/reperfusion injury].[Pubmed:18610834]
Mol Biol (Mosk). 2008 Mar-Apr;42(2):258-64.
To investigate the effect of E-64d, a selective inhibitor of calpain, on the expression of calpain and calpastatin in rat retina subject to ischemia/reperfusion injury (IRI). An animal model of retinal IRI was set up by increasing the intraocular pressure (110 mmHg) of a rat eye for 1 h. The retinal thickness and morphologic changes were detected by histology. The protein expression of m-calpain (a calpain isoform) in the retina was assessed by immunohistochemistry and Western blot assay. The mRNA of m-calpain as well as calpastatin (an endogenous protein inhibitor of calpain) in the retina was assessed by RT-PCR, and the ratio of m-calpain/calpastatin was then calculated. To evaluate the effect of E-64d on the expression of calpain, the drug (5 microl of 100 microM) was injected intravitreously immediately after IRI. There were retinal edematous changes, particularly in the inner plexiform layer after IRI. The protein expression of m-calpain in the retina was increased 24h after IRI, an effect that was inhibited by E-64d (P < 0.05). The mRNA expression of m-calpain and calpastatin was also increased 24 h and 3 h after IRI, respectively. Neither m-calpain nor calpastatin mRNA expression was influenced by E-64d (P > 0.05). The mRNA ratio of m-calpain to calpastatin was increased at the 6 h, 24 h and 72 h after IRI, and only at 24 h the increase of the ratio of m-calpain to calpastatin was inhibited by E-64d (P < 0.05). In the rat retina of IRI, E-64d inhibits the increase of m-calpain protein expression, as well as the mRNA ratio increase of m-calpain to calpastatin. E-64d also inhibited the retinal damage induced by IRI, suggesting a role for E-64d in the protection of the retinal apoptosis induced by IRI.
Detecting autophagy in Arabidopsis roots by membrane-permeable cysteine protease inhibitor E-64d and endocytosis tracer FM4-64.[Pubmed:22105025]
Plant Signal Behav. 2011 Dec;6(12):1946-9.
Autophagy is the process by which cells degrade their own components in lysosomes or vacuoles. Autophagy in tobacco BY-2 cells cultured in sucrose-free medium takes place in formed, autolysosomes in the presence of a cysteine protease inhibitor. The autolysosomes in BY-2 cells are located in the endocytotic pathway and thus can be stained with fluorescent endocytosis marker FM4-64. In the present study, in order to detect autophagy in the root cells of Arabidopsis, we incubated root tips from Arabidopsis seedlings in culture medium containing the membrane-permeable cysteine protease inhibitor E-64d and FM4-64, and examined whether autolysosomes stained with FM4-64 are accumulated. The results suggest that autophagy accompanying the formation of autolysosomes also occurs in Arabidopsis root cells. Such autophagy appeared to occur constitutively in the root cells in nutrient-sufficient culture medium. Even in atg5 mutants in which an autophagy-related gene is disrupted, accumulation of the structures stained with FM4-64, which likely correspond to autolysosomes, was seen although at lower level than in wild type roots.
Expression profiles of hippocampal regenerative sprouting-related genes and their regulation by E-64d in a developmental rat model of penicillin-induced recurrent epilepticus.[Pubmed:23266720]
Toxicol Lett. 2013 Feb 27;217(2):162-9.
E-64d (a calpain and autophagy inhibitor) has previously been shown safe for the treatment of Alzheimer's disease in humans. In the present study, the potential protective mechanism of E-64d on hippocampal aberrant mossy fiber sprouting was examined in a developmental rat model of penicillin-induced recurrent epilepticus. A seizure was induced by penicillin every other day in Sprague-Dawley rats from postnatal day 21 (P21). The rats were randomly assigned into the control group (CONT1), the control plus E-64d (CONT2), the seizure group (EXP1) and the seizure plus E-64d (EXP2). On P51, mossy fiber sprouting and related gene expression in hippocampus were assessed by Timm staining and real-time RT-PCR methods, respectively. To validate the RT-PCR results, western blot analysis was performed on selected genes. E-64d obviously suppressed the aberrant mossy fiber sprouting in the supragranular region of dentate gyrus and CA3 subfield of hippocampus. Among the total twelve genes, six genes were strongly up- (MT-3, ACAT1, clusterin and ApoE) or down- (ZnT-1 and PRG-3) regulated by developmental seizures (EXP1) compared with that in the CONT1. Up-regulation of ApoE and Clusterin was blocked by pretreatment with E-64d both in mRNA and protein levels. Further, E-64d-pretreated seizure rats (EXP2) showed a significant downregulation of mRNA expression of PRG-1, PRG-3 and PRG-5, cathepsin B and ApoE, as well as up-regulated nSMase and ANX7 in hippocampus when compared with EXP1 rats. The results of the present study suggest that E-64d, an elective inhibitor of calpain and autophagy, is potentially useful in the treatment of developmental seizure-induced brain damage both by regulating abnormal zinc signal transduction and through the modulation of altered lipid metabolism via ApoE/clusterin pathway in hippocampus.
Long-term expression of zinc transporters in hippocampus following penicillin-induced developmental seizures and its regulation by E-64d.[Pubmed:27347040]
Exp Ther Med. 2016 Jul;12(1):208-214.
Autophagy has been shown to be involved in the pathophysiology of developmental seizure-induced brain damage. The present study aimed to examine whether E-64d, an autophagy inhibitor, was able to facilitate developmental seizure-induced hippocampal mossy fiber sprouting, in particular sprouting-associated zinc transporter signals. Recurrent seizures were induced by penicillin every other day in Sprague-Dawley rats from postnatal day 21 (P21). Rats were randomly assigned into the control group (CONT), recurrent seizure group (RS) and the seizure plus E-64d group (E64D). The expression levels of beclin-1 and B-cell lymphoma 2 were analyzed at 1.5, 3, 6 and 24 h after the last seizures using western blot analysis. At P51, mossy fiber sprouting and the mRNA expression levels of zinc transporter 2 (ZnT-2), ZnT-4, ZnT-5, ZnT-6, ZnT-7, divalent cation transporter 1, Zrt-Irt-like protein 6 (ZIP-6), ZIP-7, cathepsin D and cathepsin L in the rat hippocampus were assessed using Timm staining and reverse transcription-quantitative polymerase chain reaction analysis, respectively. Reduced hippocampal mossy fiber sprouting were detected in the E-64d-treated rats compared with the non-treated control. In parallel with these observations, there was a marked reduction in the mRNA expression levels of ZnT-4 at P51 in the E-64d-treated rat hippocampus compared with the non-treated seizure group. Linear correlation analysis showed significant inter-relationship among ZIP-7, ZnT-4, ZnT-5, ZnT-7, cathepsin D and cathepsin L. These results indicate that the ZnT-4/ZIP-7/cathepsin signaling pathway serves a crucial function in the neuroprotective effects of E-64d. Thus, E-64d may offer a novel strategy for the development of therapeutic interventions for developmental seizure-induced brain damage.
Autophagy upregulation by inhibitors of caspase-3 and mTOR enhances radiotherapy in a mouse model of lung cancer.[Pubmed:18424912]
Autophagy. 2008 Jul;4(5):659-68. Epub 2008 Apr 7.
Autophagy has been reported to be increased in irradiated cancer cells resistant to various apoptotic stimuli. We therefore hypothesized that induction of autophagy via mTOR inhibition could enhance radiosensitization in apoptosis-inhibited H460 lung cancer cells in vitro and in a lung cancer xenograft model. To test this hypothesis, combinations of Z-DEVD (caspase-3 inhibitor), RAD001 (mTOR inhibitor) and irradiation were tested in cell and mouse models. The combination of Z-DEVD and RAD001 more potently radiosensitized H460 cells than individual treatment alone. The enhancement in radiation response was not only evident in clonogenic survival assays, but also was demonstrated through markedly reduced tumor growth, cellular proliferation (Ki67 staining), apoptosis (TUNEL staining) and angiogenesis (vWF staining) in vivo. Additionally, upregulation of autophagy as measured by increased GFP-LC3-tagged autophagosome formation accompanied the noted radiosensitization in vitro and in vivo. The greatest induction of autophagy and associated radiation toxicity was exhibited in the tri-modality treatment group. Autophagy marker, LC-3-II, was reduced by 3-methyladenine (3-MA), a known inhibitor of autophagy, but further increased by the addition of lysosomal protease inhibitors (pepstatin A and E64d), demonstrating that there is autophagic induction through type III PI3 kinase during the combined therapy. Knocking down of ATG5 and beclin-1, two essential autophagic molecules, resulted in radiation resistance of lung cancer cells. Our report suggests that combined inhibition of apoptosis and mTOR during radiotherapy is a potential therapeutic strategy to enhance radiation therapy in patients with non-small cell lung cancer.
Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation.[Pubmed:17942897]
Cancer Res. 2007 Oct 15;67(20):9677-84.
Several types of cancer cells, including colorectal cancer-derived cell lines, show austerity, the resistance to nutrient starvation, but exactly how cancer cells obtain energy sources under conditions in which their external nutrient supply is extremely limited remains to be clarified. Because autophagy is a catabolic process by which cells supply amino acids from self-digested organelles, cancer cells are likely to use autophagy to obtain amino acids as alternative energy sources. Amino acid deprivation-induced autophagy was assessed in DLD-1 and other colorectal cancer-derived cell lines. The autophagosome-incorporated LC3-II protein level increased after treatment with a combination of autolysosome inhibitors, which interferes with the consumption of autophagosomes. Autophagosome formation was also morphologically confirmed using ectopically expressed green fluorescent protein-LC3 fusion proteins in DLD-1 and SW480 cells. These data suggest that autophagosomes were actively produced and promptly consumed in colorectal cancer cells under nutrient starvation. Autolysosome inhibitors and 3-methyl adenine, which suppresses autophagosome formation, remarkably enhanced apoptosis under amino acid-deprived and glucose-deprived condition. Similar results were obtained in the cells with decreased ATG7 level by the RNA interference. These data suggest that autophagy is pivotal for the survival of colorectal cancer cells that have acquired austerity. Furthermore, autophagosome formation was seen only in the tumor cells but not in the adjacent noncancerous epithelial cells of colorectal cancer specimens. Taken together, autophagy is activated in colorectal cancers in vitro and in vivo, and autophagy may contribute to the survival of the cancer cells in their microenvironment.
Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy.[Pubmed:16874052]
Autophagy. 2005 Jul;1(2):84-91. Epub 2005 Jul 31.
During starvation-induced autophagy in mammals, autophagosomes form and fuse with lysosomes, leading to the degradation of the intra-autophagosomal contents by lysosomal proteases. During the formation of autophagosomes, LC3 is lipidated, and this LC3-phospholipid conjugate (LC3-II) is localized on autophagosomes and autolysosomes. While intra-autophagosomal LC3-II may be degraded by lysosomal hydrolases, recent studies have regarded LC3-II accumulation as marker of autophagy. The effect of lysosomal turnover of endogenous LC3-II in this process, however, has not been considered. We therefore investigated the lysosomal turnover of endogenous LC3-II during starvation-induced autophagy using E64d and pepstatin A, which inhibit lysosomal proteases, including cathepsins B, D and L. We found that endogenous LC3-II significantly accumulated in the presence of E64d and pepstatin A under starvation conditions, increasing about 3.5 fold in HEK293 cells and about 6.7 fold in HeLa cells compared with that in their absence, whereas the amount of LC3-II in their absence is cell-line dependent. Morphological analyses indicated that endogenous LC3-positive puncta and autolysosomes increased in HeLa cells under starvation conditions in the presence of these inhibitors. These results indicate that endogenous LC3-II is considerably degraded by lysosomal hydrolases after formation of autolysosomes, and suggest that lysosomal turnover, not a transient amount, of this protein reflects starvation-induced autophagic activity.
Inhibition of cysteine proteinases in lysosomes and whole cells.[Pubmed:1637341]
Biochem J. 1992 Jul 15;285 ( Pt 2):495-502.
Inhibitors of cysteine proteinases have been used extensively to dissect the roles of these proteinases in cells. Surprisingly though, little work has been performed to demonstrate unequivocally that the inhibitors reach and inactivate their target proteinases in cell culture or in vivo. In the present study, the permeability of lysosomes and whole cells has been studied. Benzyloxycarbonyl (Z)-[125I]iodo-Tyr-Ala-diazomethane (CHN2), an inhibitor of cathepsins L and B, has been shown to label active forms of these enzymes in lysosomes and whole cells. The ability of other cysteine proteinase inhibitors to block this labelling has been used to indicate the permeation of these compounds. All the inhibitors were able to block labelling by Z-[125I]iodo-Tyr-Ala-CHN2 in lysosomal extracts. In intact lysosomes or cells, however, only N-[N-(L-3-trans-ethoxycarbonyloxirane-2-carbonyl)-L-leucyl]-3- methylbutylamine ('E-64d') Z-Tyr-Ala-CHN2, Z-Phe-Ala-CHN2 and Z-Phe-Phe-CHN2 were able to block labelling by Z-[125I]iodo-Tyr-Ala-CHN2. N-[N-(L-3-trans-Carboxyoxirane-2-carbonyl)-L-leucyl]amino-4-gua nidinobutane (E-64) and leupeptin were unable to block labelling by Z-[125I]iodo-Tyr-Ala-CHN2 in lysosomes or in cells. The ability to block labelling in lysosomes is an indication of the ability of the inhibitor to diffuse across membranes. Thus E-64 and leupeptin do not readily permeate membranes and therefore their uptake into cells probably only occurs via pinocytosis.


