EichlerialactoneCAS# 2202-01-9 |
Quality Control & MSDS
Number of papers citing our products

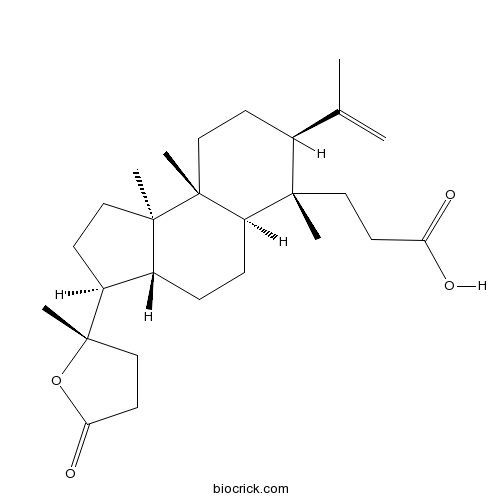
Chemical structure

3D structure
| Cas No. | 2202-01-9 | SDF | Download SDF |
| PubChem ID | 76313961 | Appearance | Powder |
| Formula | C27H42O4 | M.Wt | 430.6 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[(3S,3aR,5aR,6S,7S,9aR,9bR)-6,9a,9b-trimethyl-3-[(2S)-2-methyl-5-oxooxolan-2-yl]-7-prop-1-en-2-yl-1,2,3,3a,4,5,5a,7,8,9-decahydrocyclopenta[a]naphthalen-6-yl]propanoic acid | ||
| SMILES | CC(=C)C1CCC2(C(C1(C)CCC(=O)O)CCC3C2(CCC3C4(CCC(=O)O4)C)C)C | ||
| Standard InChIKey | LEKUPXHLKIIVCR-FAKJQIDCSA-N | ||
| Standard InChI | InChI=1S/C27H42O4/c1-17(2)18-9-15-26(5)21(24(18,3)13-11-22(28)29)8-7-19-20(10-14-25(19,26)4)27(6)16-12-23(30)31-27/h18-21H,1,7-16H2,2-6H3,(H,28,29)/t18-,19+,20-,21+,24-,25+,26+,27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Eichlerialactone and ethyl eichlerianoate show antimycobacterial activity against mycobacterium tuberculosis H37Ra, with minimum inhibitory concentration in the range of 25–50ug/mL. 2. Eichlerialactone is weakly cytotoxic to a breast cancer (BC) cell line. 3. Eichlerialactone exhibits good antibacterial activity against both the Gram-positive pathogens. |
| Targets | Antifection |

Eichlerialactone Dilution Calculator

Eichlerialactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3223 mL | 11.6117 mL | 23.2234 mL | 46.4468 mL | 58.0585 mL |
| 5 mM | 0.4645 mL | 2.3223 mL | 4.6447 mL | 9.2894 mL | 11.6117 mL |
| 10 mM | 0.2322 mL | 1.1612 mL | 2.3223 mL | 4.6447 mL | 5.8059 mL |
| 50 mM | 0.0464 mL | 0.2322 mL | 0.4645 mL | 0.9289 mL | 1.1612 mL |
| 100 mM | 0.0232 mL | 0.1161 mL | 0.2322 mL | 0.4645 mL | 0.5806 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Vincetoxicoside B
Catalog No.:BCN2864
CAS No.:22007-72-3
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- Ixabepilone
Catalog No.:BCC1666
CAS No.:219989-84-1
- Fmoc-β-Homo-Tyr(tBu)-OH
Catalog No.:BCC2621
CAS No.:219967-69-8
- Isoescin IB
Catalog No.:BCN2969
CAS No.:219944-46-4
- Isoescin IA
Catalog No.:BCN2968
CAS No.:219944-39-5
- 3-Methyl-1-(2-piperidinophenyl)butylamine N-acetylglutamate salt
Catalog No.:BCC8635
CAS No.:219921-94-5
- 5,8,9,14-Tetraacetoxy-3-benzoyloxy-10,15-dihydroxypepluane
Catalog No.:BCN7657
CAS No.:219916-77-5
- MPEP Hydrochloride
Catalog No.:BCC1777
CAS No.:219911-35-0
- 2,2',3'-Trihydroxy-4,6-dimethoxybenzophenone
Catalog No.:BCN1488
CAS No.:219861-73-1
- Escitalopram Oxalate
Catalog No.:BCC5040
CAS No.:219861-08-2
- O-Methyldauricine
Catalog No.:BCC8225
CAS No.:2202-17-7
- Triptohairic acid
Catalog No.:BCN8060
CAS No.:220209-71-2
- Cinnamamide
Catalog No.:BCN4942
CAS No.:22031-64-7
- 5-[2-[Tert-butyl(dimethyl)silyl]oxyethyl]-2,2-dimethyl-3a,6a-dihydrofuro[2,3-d][1,3]dioxol-6-one
Catalog No.:BCC8592
CAS No.:220328-03-0
- 3,11,12-Trihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1487
CAS No.:220328-04-1
- TRO 19622
Catalog No.:BCC5288
CAS No.:22033-87-0
- DH 97
Catalog No.:BCC6973
CAS No.:220339-00-4
- 5-Epicanadensene
Catalog No.:BCN7349
CAS No.:220384-17-8
- ShK-Dap22
Catalog No.:BCC5990
CAS No.:220384-25-8
- Polycephalin C
Catalog No.:BCN1852
CAS No.:220422-37-7
- Kaempferol 5-methyl ether
Catalog No.:BCN3426
CAS No.:22044-80-0
- Leptomerine
Catalog No.:BCN1486
CAS No.:22048-97-1
Cytotoxicity and Synergistic Effect of the Constituents from Roots of Aglaia odorata (Meliaceae).[Pubmed:25742723]
Nat Prod Res. 2016;30(4):433-7.
Twelve compounds were isolated from the roots of Aglaia odorata. Their structures were established on the basis of NMR and MS data as rocaglaol (1), rocaglamide (2), Eichlerialactone (3), sapelins A (4), isofouquierone (5), eichlerianic acid (6), shoreic acid (7), agladupol E (8), 3-epimeliantriol (9), cleomiscosins B (10), 2beta,3beta-dihydroxy-5alpha-pregnane-16-one (11) and beta-D-glucopyranos-1-yl N-methylpyrrole-2-carboxylate (12). Among them, compounds 1 and 2 showed significant cytotoxicity against human cancer cell (HL-60, SMMC-7721, A-549, MCF-7 and SW480) with IC50 values of 0.007-0.095 muM, while compounds 3-5 and 10 and 11 showed moderate to no cytotoxicity (IC50 0.43 to values >40 muM). Compound 6 showed only weak cytotoxicity (IC50 6.87 to >40 muM) and its epmier 7 was completely inactivite (IC50>40 muM) in the assay. However, potent synergistic effect was observed when the molar ratio of 6 to 7 is between 4:1 and 1:1.
A new sesquiterpene and other terpenoid constituents of Chisocheton penduliflorus.[Pubmed:18277603]
Arch Pharm Res. 2008 Jan;31(1):21-7.
A new aromadendrane sesquiterpene, allo-aromadendrane-10alpha, 14-diol (3), was isolated from Chisocheton penduliflorus (Meliaceae), along with two known sesquiterpenes: allo-aromadendrane-10beta, 14-diol (2) and allo-aromadendrane-10beta, 13, 14-triol (7). Six dammarane triterpenoids, including cabraleadiol (1), Eichlerialactone (4), cabraleahydroxylactone (5), cabralealactone (6), hollongdione (8) and dammaradienone (9), the coumarins scoparone and scopoletin, and vanillic acid were also isolated from the wood and leaves of this plant. Compounds 1-7 displayed antimycobacterial activity against Mycobacterium tuberculosis. Compounds 1, 4, 5 and 6 were weakly cytotoxic to a breast cancer (BC) cell line; whereas, compound 6 was moderately active against a small-cell lung cancer (NCI-H187) cell line.
Biologically active constituents of Aglaia erythrosperma.[Pubmed:22011221]
Nat Prod Res. 2011 Oct;25(17):1621-8.
From the fruits and leaves of Aglaia erythrosperma (Meliaceae), 10 chemical constituents were isolated and identified, i.e. the dammarane triterpenoids cabraleadiol (1), cabraleahydroxylactone (2), ethyl eichlerianoate (3), Eichlerialactone (4), aglinin A (5), cabralealactone (6), the aglaialactone 5,6-desmethylenedioxy-5-methoxy-aglalactone (7), the flavagline 4'-demethoxy-3',4'-methylenedioxy-methyl rocaglate (8) and two coumarins: scoparone and scopoletin. Flavagline 8 exhibited antimalarial activity with an IC(50) value of 7.30 microg mL(-1) and was strongly cytotoxic against small cell lung cancer (NCI-H187), epidermoid carcinoma (KB) and breast cancer (BC) cell lines, with IC(50) values of 2.17, 2.10 and 0.11 microg mL(-1), respectively. Aglinin A (5) displayed moderate cytotoxicity against all the three cancer cell lines, whereas ethyl eichlerianoate (3), cabralealactone (6) and the aglaialactone 7 were exclusively cytotoxic to NCI-H187 cell line. Cabraleahydroxylactone (2) showed antiviral activity against herpes simplex virus type-1 with an IC(50) value of 3.20 microg mL(-1), in comparison with the standard acyclovir (IC(50) = 1.90 microg mL(-1)). When tested for antimycobacterial activity against Mycobacterium tuberculosis H(37)Ra, compounds 1-4 and 6-8 displayed minimum inhibitory concentration in the range of 25-50 microg mL(-1).


