Endomorphin-2Potent and selective μ agonist CAS# 141801-26-5 |
- alpha-Endorphin
Catalog No.:BCC1010
CAS No.:59004-96-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 141801-26-5 | SDF | Download SDF |
| PubChem ID | 5311081 | Appearance | Powder |
| Formula | C32H37N5O5 | M.Wt | 571.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 0.40 mg/ml in water | ||
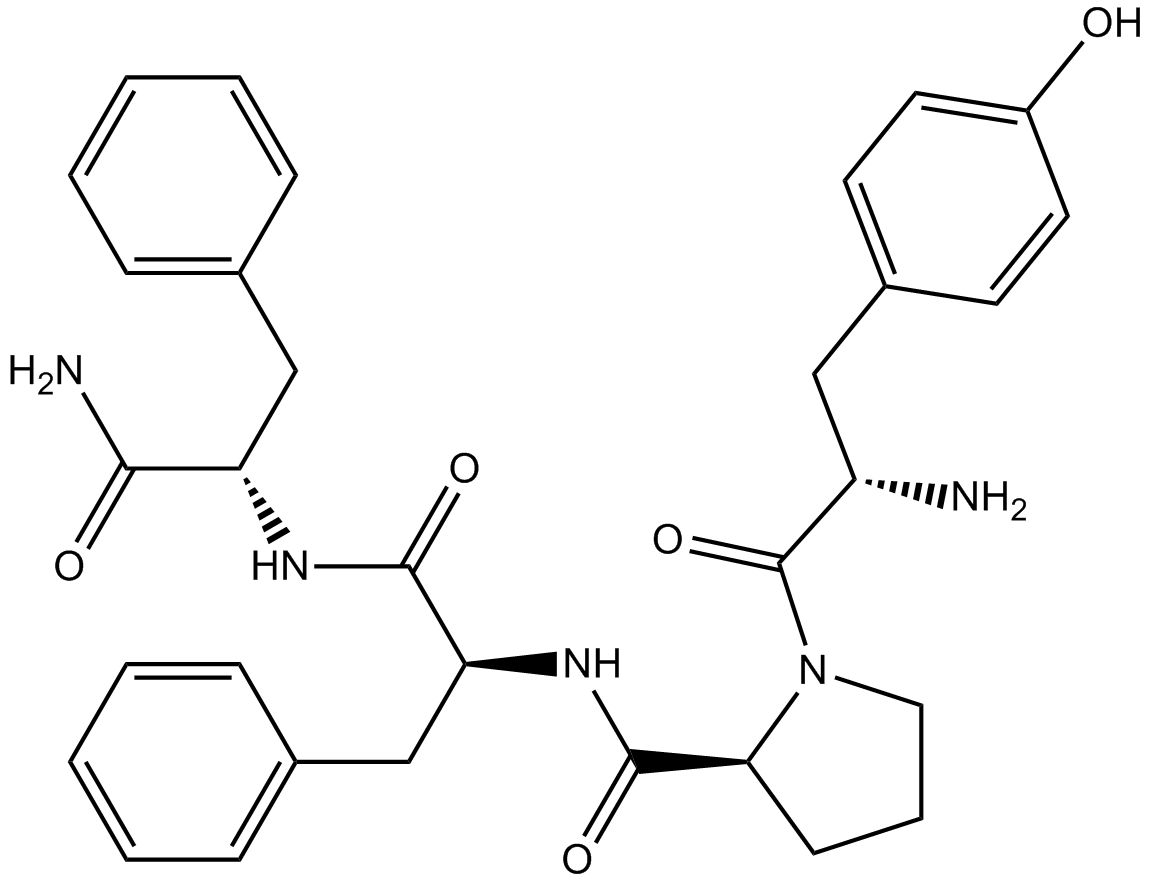
| Sequence | YPFF (Modifications: Phe-3 = C-terminal amide) | ||
| Chemical Name | (2S)-1-[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]-N-[(2S)-1-[[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]pyrrolidine-2-carboxamide | ||
| SMILES | C1CC(N(C1)C(=O)C(CC2=CC=C(C=C2)O)N)C(=O)NC(CC3=CC=CC=C3)C(=O)NC(CC4=CC=CC=C4)C(=O)N | ||
| Standard InChIKey | XIJHWXXXIMEHKW-LJWNLINESA-N | ||
| Standard InChI | InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous peptide with extremely high affinity and selectivity for μ-opioid receptors (with Ki values of 0.69, 9233 and 5240 nM for μ, δ and κ respectively). |

Endomorphin-2 Dilution Calculator

Endomorphin-2 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Stauntosaponin A
Catalog No.:BCN6966
CAS No.:1417887-91-2
- Albatrelin C
Catalog No.:BCN7591
CAS No.:1417805-17-4
- Albatrelin A
Catalog No.:BCN7626
CAS No.:1417805-15-2
- Exendin-4
Catalog No.:BCC1568
CAS No.:141758-74-9
- SDZ SER 082 fumarate
Catalog No.:BCC6994
CAS No.:1417343-80-6
- MM-102
Catalog No.:BCC4551
CAS No.:1417329-24-8
- Exenatide acetate
Catalog No.:BCN8514
CAS No.:141732-76-5
- H-DL-Phe(4-Cl)-OMe.HCl
Catalog No.:BCC3175
CAS No.:14173-40-1
- H-Phe(4-Cl)-OH
Catalog No.:BCC3171
CAS No.:14173-39-8
- RI-2
Catalog No.:BCC6424
CAS No.:1417162-36-7
- Faropenem daloxate
Catalog No.:BCC1571
CAS No.:141702-36-5
- Galanin (2-29) (rat)
Catalog No.:BCC5763
CAS No.:141696-11-9
- NMS-873
Catalog No.:BCC4977
CAS No.:1418013-75-8
- LMK 235
Catalog No.:BCC2421
CAS No.:1418033-25-6
- Minaxin C
Catalog No.:BCN7656
CAS No.:1418150-06-7
- EI1
Catalog No.:BCC4044
CAS No.:1418308-27-6
- Magnoloside D
Catalog No.:BCN8062
CAS No.:1418309-03-1
- Fmoc-Cys(pMeOBzl)-OH
Catalog No.:BCC3477
CAS No.:141892-41-3
- H-HoTyr-OH.HBr
Catalog No.:BCC3245
CAS No.:141899-12-9
- 4-Hydroxyphenylacetonitrile
Catalog No.:BCN6905
CAS No.:14191-95-8
- Pimentol
Catalog No.:BCN2714
CAS No.:141913-95-3
- Ginsenoyne K
Catalog No.:BCN3953
CAS No.:141947-42-4
- Ginsenoside Rg3
Catalog No.:BCN1068
CAS No.:14197-60-5
- 14-Deoxy-11,12-didehydroandrographiside
Catalog No.:BCN1572
CAS No.:141973-41-3
Correction for "Synthesis of Mixed Opioid Affinity Cyclic Endomorphin-2 Analogues with Fluorinated Phenylalanines".[Pubmed:27326343]
ACS Med Chem Lett. 2016 May 9;7(6):652.
[This corrects the article DOI: 10.1021/acsmedchemlett.5b00056.].
Endomorphin-2 Inhibition of Substance P Signaling within Lamina I of the Spinal Cord Is Impaired in Diabetic Neuropathic Pain Rats.[Pubmed:28119567]
Front Mol Neurosci. 2017 Jan 10;9:167.
Opiate analgesia in the spinal cord is impaired in diabetic neuropathic pain (DNP), but until now the reason is unknown. We hypothesized that it resulted from a decreased inhibition of substance P (SP) signaling within the dorsal horn of the spinal cord. To investigate this possibility, we evaluated the effects of Endomorphin-2 (EM2), an endogenous ligand of the mu-opioid receptor (MOR), on SP release within lamina I of the spinal dorsal horn (SDH) in rats with DNP. We established the DNP rat model and compared the analgesic efficacy of EM2 between inflammation pain and DNP rat models. Behavioral results suggested that the analgesic efficacy of EM2 was compromised in the condition of painful diabetic neuropathy. Then, we measured presynaptic SP release induced by different stimulating modalities via neurokinin-1 receptor (NK1R) internalization. Although there was no significant change in basal and evoked SP release between control and DNP rats, EM2 failed to inhibit SP release by noxious mechanical and thermal stimuli in DNP but not in control and inflammation pain model. We also observed that EM2 decreased the number of FOS-positive neurons within lamina I of the SDH but did not change the amount of FOS/NK1R double-labeled neurons. Finally, we identified a remarkable decrease in MORs within the primary afferent fibers and dorsal root ganglion (DRG) neurons by Western blot (WB) and immunohistochemistry (IHC). Taken together, these data suggest that reduced presynaptic MOR expression might account for the loss of the inhibitory effect of EM2 on SP signaling, which might be one of the neurobiological foundations for decreased opioid efficacy in the treatment of DNP.
Decreased Endomorphin-2 and mu-Opioid Receptor in the Spinal Cord Are Associated with Painful Diabetic Neuropathy.[Pubmed:27656127]
Front Mol Neurosci. 2016 Sep 7;9:80.
Painful diabetic neuropathy (PDN) is one of the most common complications in the early stage of diabetes mellitus (DM). Endomorphin-2 (EM2) selectively activates the mu-opioid receptor (MOR) and subsequently induces antinociceptive effects in the spinal dorsal horn. However, the effects of EM2-MOR in PDN have not yet been clarified in the spinal dorsal horn. Therefore, we aimed to explore the role of EM2-MOR in the pathogenesis of PDN. The main findings were the following: (1) streptozotocin (STZ)-induced diabetic rats exhibited hyperglycemia, body weight loss and mechanical allodynia; (2) in the spinal dorsal horn, the expression levels of EM2 and MOR decreased in diabetic rats; (3) EM2 protein concentrations decreased in the brain, lumbar spinal cord and cerebrospinal fluid (CSF) in diabetic rats but were unchanged in the plasma; (4) the frequency but not the amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) was significantly higher in diabetic rats than in control rats; and (5) intrathecal injection of EM2 for 14 days in the early stage of PDN partially alleviated mechanical allodynia and reduced MOR expression in diabetic rats. Our results demonstrate that the EM2-MOR signal may be involved in the early stage of PDN.
Endomorphin-2 analogs with C-terminal esterification produce potent systemic antinociception with reduced tolerance and gastrointestinal side effects.[Pubmed:28042019]
Neuropharmacology. 2017 Apr;116:98-109.
C-terminal esterification of opioid peptides may change their opioid activities due to the modified physicochemical properties. In the present study, the pharmacological activities of C-terminal esterified Endomorphin-2 (EM-2) analogs 1-3 were characterized by in vitro metabolic stability and octanol/buffer distribution assays. Also, the antinociceptive profiles in the radiant heat paw withdrawal test and related side effects of these analogs were determined. Our results showed that all three analogs significantly increased the metabolic stability and lipophilicity. Moreover, analogs 1-3 displayed potent antinociceptive activities after intracerebroventricular (i.c.v.) administration. Analogs 1 and 3 exhibited about 2-fold higher antinociception than EM-2, and differential opioid mechanisms were involved. In addition, EM-2 at 50 mumol/kg failed to produce any significant antinociceptive activity after subcutaneous (s.c.) administration, whereas equimolar dose of analogs 1-3 produced significant analgesic effects. Analog 3 showed the highest antinociceptive activity after systemic administration, which was consistent with its in vitro stability and lipophilicity. We further evaluated the antinociceptive tolerance of analogs 1-3. In acute tolerance test, analogs 1-3 shifted the dose-response curves rightward by only 1.4-3.2 fold as determined by tolerance ratio, whereas EM-2 by 5.6-fold, demonstrating reduced antinociceptive tolerance. Also, analogs 1 and 2 decreased chronic antinociceptive tolerance by central and peripheral administration of drugs. In particular, analogs 3 displayed insignificant chronic antinociceptive tolerance. Furthermore, analogs 1-3 were less prone to induce gastrointestinal side effects at analgesic doses. The present investigation gave the evidence that C-terminal esterified modifications of EM-2 will facilitate the development of novel opioid analgesics with reduced side effects.
Pharmacological characterization of endomorphin-1 and endomorphin-2 in mouse brain.[Pubmed:9694962]
J Pharmacol Exp Ther. 1998 Aug;286(2):1007-13.
The recently isolated peptides endomorphin-1 and Endomorphin-2 have been suggested to be the endogenous ligands for the mu receptor. In traditional opioid receptor binding assays in mouse brain homogenates, both endomorphin-1 and Endomorphin-2 competed both mu1 and mu2 receptor sites quite potently. Neither compound had appreciable affinity for either delta or kappa1 receptors, confirming an earlier report. However, the two endomorphins displayed reasonable affinities for kappa3 binding sites, with Ki values between 20 and 30 nM. Both endomorphins competed 3H-[D-Ala2, MePhe4,Gly(ol)5] enkephalin binding to MOR-1 receptors expressed in CHO cells with high affinity. In mouse brain homogenates 125I-endomorphin-1 and 125I-Endomorphin-2 binding was selectively competed by mu ligands. 125I-Endomorphin-1 and 125I-Endomorphin-2 also labeled MOR-1 receptors expressed in CHO cells with high affinity. Autoradiography of the two 125I-labeled endomorphins demonstrated regional patterns in the brain similar to those previously observed for mu drugs. Pharmacologically, the endomorphins were potent analgesics. Although they were equipotent supraspinally, endomorphin-1 was more potent spinally. Endomorphin analgesia was effectively blocked by naloxone, as well as the mu-selective antagonists beta-funaltrexamine and naloxonazine. In CXBK mice, which are insensitive to supraspinal morphine, neither endomorphin was active, consistent with a mu mechanism of action. Finally, the endomorphins inhibited gastrointestinal transit. In conclusion, these results support the mu selectivity of these agents.
Differential effects of endomorphin-1, endomorphin-2, and Tyr-W-MIF-1 on activation of G-proteins in SH-SY5Y human neuroblastoma membranes.[Pubmed:9622031]
Peptides. 1998;19(4):749-53.
Endomorphin-1 (Tyr-Pro-Trp-Phe-NH2) and Endomorphin-2 (Tyr-Pro-Phe-Phe-NH2), peptides recently isolated from bovine and human brain, have high affinity and selectivity for mu opiate receptors. They share sequence similarity with the endogenous opiate-modulating peptide Tyr-W-MIF-1 (Tyr-Pro-Trp-Gly-NH2). The efficacies of these endogenous peptides and of the enkephalin analog DAMGO were compared by measuring their effects on the binding of guanosine-5'-O-(-gamma-[35S]thio)triphosphate ([35S]GTPgammaS) to G-proteins in membranes from SH-SYSY human neuroblastoma cells. DAMGO, endomorphin-1, and Endomorphin-2 stimulated [35S]GTPgammaS binding dose dependently, with maximal effects of 60 +/- 9%, 47 +/- 9%, and 43 +/- 6% stimulation above basal and ED50 of 49 +/- 8 nM, 38 +/- 8 nM, and 64 +/- 13 nM, respectively. Tyr-W-MIF-1 showed only a small stimulation of binding (5% stimulation above basal, ED50 = 2 microM). When given in combination with the other opioids, however, Tyr-W-MIF-1 attenuated their ability to activate G-proteins. Thus, the endogenous opioids endomorphin-1 and Endomorphin-2 activate G-proteins similarly to the synthetic agonist DAMGO, but the structurally similar peptide Tyr-W-MIF-1 produces only minimal stimulation of G-proteins.
Distinct inhibitory effects of spinal endomorphin-1 and endomorphin-2 on evoked dorsal horn neuronal responses in the rat.[Pubmed:9422796]
Br J Pharmacol. 1997 Dec;122(8):1537-9.
Intrathecal endomorphin-1 and Endomorphin-2 (0.25-50 micrograms) dose-relatedly reduced all components of electrical evoked C-fibre responses of spinal neurones. These effects were partially reversed by naloxone. Endomorphin-1, but not Endomorphin-2, dose-relatedly reduced the A beta-fibre evoked responses. Peak inhibitory effects of endomorphin-1 and -2 were at 15-20 min post-administration. Thus spinal Endomorphin-2 had selective effects on noxious responses, whereas endomorphin-1 was non-selective.
A potent and selective endogenous agonist for the mu-opiate receptor.[Pubmed:9087409]
Nature. 1997 Apr 3;386(6624):499-502.
Peptides have been identified in mammalian brain that are considered to be endogenous agonists for the delta (enkephalins) and kappa (dynorphins) opiate receptors, but none has been found to have any preference for the mu receptor. Because morphine and other compounds that are clinically useful and open to abuse act primarily at the mu receptor, it could be important to identify endogenous peptides specific for this site. Here we report the discovery and isolation from brain of such a peptide, endomorphin-1 (Tyr-Pro-Trp-Phe-NH2), which has a high affinity (Ki = 360 pM) and selectivity (4,000- and 15,000-fold preference over the delta and kappa receptors) for the mu receptor. This peptide is more effective than the mu-selective analogue DAMGO in vitro and it produces potent and prolonged analgesia in mice. A second peptide, Endomorphin-2 (Tyr-Pro-Phe-Phe-NH2), which differs by one amino acid, was also isolated. The new peptides have the highest specificity and affinity for the mu receptor of any endogenous substance so far described and they may be natural ligands for this receptor.


