Finasteride acetate5α-reductase inhibitor CAS# 222989-99-3 |
- WZ4002
Catalog No.:BCC1074
CAS No.:1213269-23-8
- AZD-9291
Catalog No.:BCC4120
CAS No.:1421373-65-0
- PD153035 hydrochloride
Catalog No.:BCC3617
CAS No.:153436-54-5
- FIIN-2
Catalog No.:BCC3974
CAS No.:1633044-56-0
- Erlotinib
Catalog No.:BCC1557
CAS No.:183321-74-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 222989-99-3 | SDF | Download SDF |
| PubChem ID | 78357778 | Appearance | Powder |
| Formula | C25H40N2O4 | M.Wt | 432.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MK-906 acetate | ||
| Solubility | Soluble in DMSO | ||
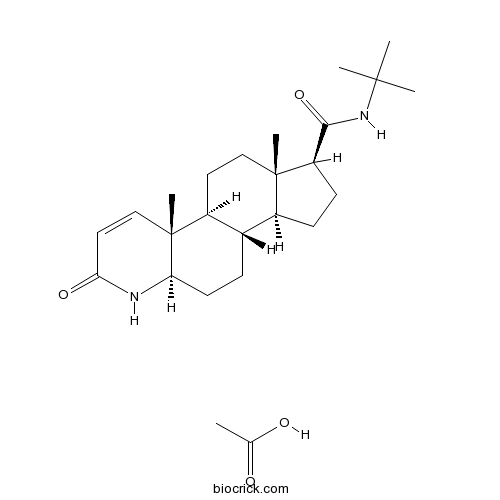
| Chemical Name | (1S,3aS,3bS,5aR,9aR,9bS,11aS)-N-tert-butyl-9a,11a-dimethyl-7-oxo-1,2,3,3a,3b,4,5,5a,6,9b,10,11-dodecahydroindeno[5,4-f]quinoline-1-carboxamide;acetic acid | ||
| SMILES | CC(=O)O.CC12CCC3C(C1CCC2C(=O)NC(C)(C)C)CCC4C3(C=CC(=O)N4)C | ||
| Standard InChIKey | CYWQSECJQBIRJR-ZNBOUQNXSA-N | ||
| Standard InChI | InChI=1S/C23H36N2O2.C2H4O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4;1-2(3)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27);1H3,(H,3,4)/t14-,15-,16-,17+,18+,22-,23+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Finasteride (acetate) is an orally active testosterone 5-alpha-reductase inhibitor.
Target: 5-alpha Reductase
Approved: 1992
Finasteride (acetate) is the acetate salt of finasteride which is a synthetic 4-azasteroid antiandrogen compound, is a specific inhibitor of steroid Type II 5α-reductase, an intracellular enzyme that converts the androgen testosterone into 5α-dihydrotestosterone (DHT). Finasteride is used in the treatment of prostate cancer, benign prostatic hyperplasia, and androgenetic alopecia (male pattern baldness). In benign prostatic hyperplasia, finasteride inhibits 5alpha-reductase activity in epithelium for Ki of 10nM, significantly lower than in stroma (Ki = 33nM) [1]. References: | |||||

Finasteride acetate Dilution Calculator

Finasteride acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3116 mL | 11.558 mL | 23.116 mL | 46.2321 mL | 57.7901 mL |
| 5 mM | 0.4623 mL | 2.3116 mL | 4.6232 mL | 9.2464 mL | 11.558 mL |
| 10 mM | 0.2312 mL | 1.1558 mL | 2.3116 mL | 4.6232 mL | 5.779 mL |
| 50 mM | 0.0462 mL | 0.2312 mL | 0.4623 mL | 0.9246 mL | 1.1558 mL |
| 100 mM | 0.0231 mL | 0.1156 mL | 0.2312 mL | 0.4623 mL | 0.5779 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Finasteride acetate(MK-906,Proscar and Propecia) is a specific inhibitor of 5α-reductase and a drug of synthetic used in the benign prostatic hyperplasia (BPH) and the male pattern baldness (MPB) [1].
In vivo, Finasteride acetate (MK-906, Proscar and Propecia) has been reported to significant reduce the concentration of DHT in prostatic tissue without side effects in pharmacologic experiments in rats and dogs. In addition, Finasteride acetate has shown the inhibition of 5α-reductase activity resulted in significant inhibition of DHT levels. Finasteride acetate has also shown the effect on reducing prostate volume in clinical experiments [1]. Finasteride acetate has been revealed to have the function to inhibit glucuronyl transferase in vitro test using rat prostate tissue [2].
References:
[1] Stoner E.The clinical development of a 5 alpha-reductase inhibitor, finasteride. J Steroid Biochem Mol Biol. 1990 Nov 20;37(3):375-8.
[2] Rittmaster RS1, Stoner E, Thompson DL, Nance D, Lasseter KC.Effect of MK-906, a specific 5 alpha-reductase inhibitor, on serum androgens and androgen conjugates in normal men. J Androl. 1989 Jul-Aug;10(4):259-62.
- Noladin ether
Catalog No.:BCC5756
CAS No.:222723-55-9
- SZL P1-41
Catalog No.:BCC8004
CAS No.:222716-34-9
- Chysin A
Catalog No.:BCN2020
CAS No.:22269-11-0
- ACBC
Catalog No.:BCC6584
CAS No.:22264-50-2
- Antidesmone
Catalog No.:BCN5058
CAS No.:222629-77-8
- Bromocriptine mesylate
Catalog No.:BCC6642
CAS No.:22260-51-1
- Tempol
Catalog No.:BCC4862
CAS No.:2226-96-2
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- Loganic acid
Catalog No.:BCN5057
CAS No.:22255-40-9
- Lucidin 3-O-glucoside
Catalog No.:BCN8249
CAS No.:22255-29-4
- Trimethoxystilbene
Catalog No.:BCN6762
CAS No.:22255-22-7
- Guaijaverin
Catalog No.:BCN5056
CAS No.:22255-13-6
- SEA0400
Catalog No.:BCC1941
CAS No.:223104-29-8
- Inolitazone
Catalog No.:BCC1652
CAS No.:223132-37-4
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- Evodol
Catalog No.:BCN5059
CAS No.:22318-10-1
- Platycodigenin
Catalog No.:BCN3183
CAS No.:22327-82-8
- Methyl ferulate
Catalog No.:BCN4023
CAS No.:22329-76-6
- 9-Hydroxy-alpha-lapachone
Catalog No.:BCN5060
CAS No.:22333-58-0
- Grandiflorenic acid
Catalog No.:BCN4670
CAS No.:22338-67-6
- Grandifloric acid
Catalog No.:BCN4669
CAS No.:22338-69-8
- Polygalacic acid
Catalog No.:BCN5898
CAS No.:22338-71-2
- FG2216
Catalog No.:BCC6402
CAS No.:223387-75-5
- GLP-2 (human)
Catalog No.:BCC5891
CAS No.:223460-79-5
Use of cyproterone acetate, finasteride, and spironolactone to treat idiopathic hirsutism.[Pubmed:12749435]
Fertil Steril. 2003 Apr;79(4):942-6.
OBJECTIVE: To compare the effectiveness of cyproterone acetate, finasteride, and spironolactone in the treatment of idiopathic hirsutism. DESIGN: Prospective randomized clinical study. SETTING: University hospital. PATIENT(S): Forty-one women (median age, 21 years [range, 18-34 years]) with idiopathic hirsutism who had requested to use an oral contraceptive. INTERVENTION(S): Patients were randomly assigned to receive cyproterone acetate (12.5 mg/d for the first 10 days of the cycle), finasteride (5 mg/d), or spironolactone (100 mg/d) for 12 months. Follow-up was done at the end of therapy. MAIN OUTCOME MEASURE(S): Ferriman-Gallwey score before treatment, at 6 and 12 months of treatment, and 1 year after the end of treatment, and androgenic profile before and after treatment. RESULT(S): At the end of therapy, the Ferriman-Gallwey score decreased by 38.9%, 38.6%, and 38.5% in patients who used cyproterone acetate, finasteride, and spironolactone, respectively. One year after therapy, the Ferriman-Gallwey score of patients who used spironolactone was significantly lower (6.74 +/- 1.41) than that of patients who used either cyproterone acetate (7.92 +/- 1.08), or finasteride (9.08 +/- 0.99). The androgenic profile did not change significantly during treatment. CONCLUSION(S): In patients with idiopathic hirsutism, the short-term results of treatment with cyproterone acetate, finasteride, and spironolactone are similar, but spironolactone is effective for a longer time.
Effects of finasteride and cyproterone acetate on hematuria associated with benign prostatic hyperplasia: a prospective, randomized, controlled study.[Pubmed:11880073]
Urology. 2002 Mar;59(3):373-7.
OBJECTIVES: To evaluate the influence of two differently acting antiandrogens, finasteride (FIN) and cyproterone acetate (CPA), on the natural history of hematuria associated with benign prostatic hyperplasia (BPH) in a prospective, randomized, controlled study. METHODS: Forty-two patients with hematuria episodes due to BPH were randomly allocated to three subgroups of 14 patients each and treated daily with either 5 mg FIN or 100 mg CPA or were placed in a watchful waiting arm. Patients were evaluated at 3-month intervals, and 40 patients had at least 1 year of follow-up. RESULTS: Four patients in the FIN group (30%) and three in the CPA group (23%) presented with recurrent hematuria. In both groups, the bleeding episodes were treated conservatively and required no hospitalization. In the control group, 8 patients (57%) presented with recurrent bleeding; in 4, the bleeding was severe and required some form of intervention (catheterization or transurethral prostatectomy). When the frequency and severity of the hematuria episodes were analyzed over time, a statistically significant difference for FIN versus control was present at 9 and 12 months (analysis of variance, P = 0.035 and P = 0.009, respectively). A similar difference was evident for CPA versus control at 9 and 12 months (P = 0.028 and P = 0.008, respectively). No statistically significant difference was present between the FIN and CPA groups. Interestingly, no statistically significant effect in bleeding recurrence for both CPA and FIN over controls was present at 3 and 6 months of follow-up. CONCLUSIONS: Both FIN and CPA seem to exert a comparable control in hematuria recurrence in patients with BPH, thus confirming the rationale behind the use of antiandrogens for such a purpose. Our results support the hypothesis that any antiandrogen, irrespective of the mode of action, would alter the natural history of BPH-associated hematuria. Interestingly, our results indicate that the speed of action of FIN may not be as rapid as previously described.
Finasteride versus cyproterone acetate-estrogen regimens in the treatment of hirsutism.[Pubmed:15464773]
Int J Gynaecol Obstet. 2004 Oct;87(1):29-33.
OBJECTIVES: To compare the clinical and hormonal effects of finasteride and a combination regimen of cyproterone acetate (CPA) plus ethinyl estradiol (EE2) in the treatment of hirsutism. METHODS: Forty hirsute women were enrolled in a prospective randomized trial. Twenty-nine had polycystic ovary syndrome (PCOS) and 11 had idiopathic hirsutism. Patients were randomly treated with finasteride (5 mg/day; n=20) or CPA plus EE2 [CPA (25 mg/day on days 5-14) plus EE2 (20 microg/day on days 5-25) n=20] for 9 months. Main outcome measurement was a reduction in hair growth. Hirsutism score and hormone levels were measured at the beginning and at the end of the study. The student t-test and Mann-Whitney U tests were used for analysis of the data. RESULTS: The modified Ferriman-Gallwey scores for hirsutism decreased significantly at the end of the study from a mean+/-SD of 23.7+/-4.4 to 11.3+/-1.5; P=<0.001 in finasteride group and from 22.3+/-4.2 to 11.4+/-1.2; P=<0.001 in CPA plus EE2 group. Improvement of hirsutism induced by the two treatment methods was similar (47.6 % vs. 51.1%; P=0.2). Treatment with CPA plus EE2 significantly decreased serum total and free T, A, DHEAS, and DHT and increased SHBG levels. Finasteride significantly increased total T but reduced DHT levels. CONCLUSION: Finasteride and CPA plus EE2 are equally effective in decreasing hirsutism, despite significantly different effects on serum hormone levels.


