Fuligorubin ACAS# 108343-55-1 |
Quality Control & MSDS
Number of papers citing our products

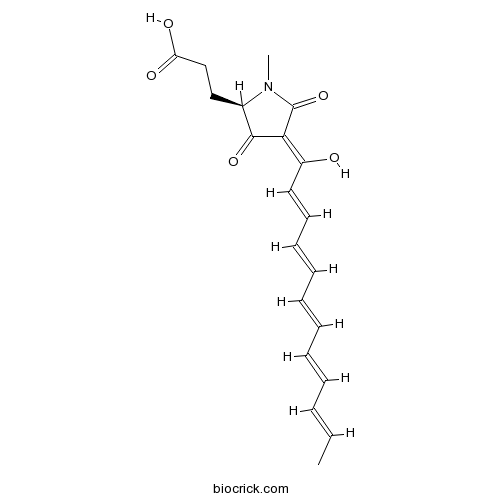
Chemical structure

3D structure
| Cas No. | 108343-55-1 | SDF | Download SDF |
| PubChem ID | 102328490 | Appearance | Red cryst. |
| Formula | C20H23NO5 | M.Wt | 357.40 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[(2R)-4-[(2E,4E,6E,8E,10E)-1-hydroxydodeca-2,4,6,8,10-pentaenylidene]-1-methyl-3,5-dioxopyrrolidin-2-yl]propanoic acid | ||
| SMILES | CC=CC=CC=CC=CC=CC(=C1C(=O)C(N(C1=O)C)CCC(=O)O)O | ||
| Standard InChIKey | VRWPPQTYMAFCLL-STUTZMFMSA-N | ||
| Standard InChI | InChI=1S/C20H23NO5/c1-3-4-5-6-7-8-9-10-11-12-16(22)18-19(25)15(13-14-17(23)24)21(2)20(18)26/h3-12,15,22H,13-14H2,1-2H3,(H,23,24)/b4-3+,6-5+,8-7+,10-9+,12-11+,18-16?/t15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Tetrahedron,1992,48(6): 1145–1174.Further reactions of t-butyl 3-oxobutanthioate and t-butyl 4-diethyl-phosphono-3-oxobutanthioate : Carbonyl coupling reactions, amination, use in the preparation of 3-acyltetramic acids and application to the total synthesis of fuligorubin A.[Reference: WebLink]
Tetrahedron Letters,1988,29(45):5829–5832.Use of t-butyl 4-diethylphosphono-3-oxobutanethioate for tetramic acid synthesis: Total synthesis of the plasmodial pigment fuligorubin A[Reference: WebLink]
|

Fuligorubin A Dilution Calculator

Fuligorubin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.798 mL | 13.9899 mL | 27.9799 mL | 55.9597 mL | 69.9496 mL |
| 5 mM | 0.5596 mL | 2.798 mL | 5.596 mL | 11.1919 mL | 13.9899 mL |
| 10 mM | 0.2798 mL | 1.399 mL | 2.798 mL | 5.596 mL | 6.995 mL |
| 50 mM | 0.056 mL | 0.2798 mL | 0.5596 mL | 1.1192 mL | 1.399 mL |
| 100 mM | 0.028 mL | 0.1399 mL | 0.2798 mL | 0.5596 mL | 0.6995 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Noradrenaline bitartrate monohydrate
Catalog No.:BCC4810
CAS No.:108341-18-0
- Ganoderic acid D
Catalog No.:BCN2437
CAS No.:108340-60-9
- Geneticin, G-418 Sulfate
Catalog No.:BCC1202
CAS No.:108321-42-2
- Fmoc-D-Asn-OH
Catalog No.:BCC3083
CAS No.:108321-39-7
- 1,7-Bis(4-hydroxyphenyl)hept-1-en-3-one
Catalog No.:BCN1629
CAS No.:1083200-79-6
- 1,7-Bis(4-hydroxyphenyl)hept-6-en-3-ol
Catalog No.:BCN1630
CAS No.:1083195-05-4
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- A 987306
Catalog No.:BCC7732
CAS No.:1082954-71-9
- LY2584702
Catalog No.:BCC6369
CAS No.:1082949-67-4
- PF-04447943
Catalog No.:BCC1850
CAS No.:1082744-20-4
- PDE-9 inhibitor
Catalog No.:BCC1842
CAS No.:1082743-70-1
- TC-S 7005
Catalog No.:BCC6189
CAS No.:1082739-92-1
- FH535
Catalog No.:BCC1573
CAS No.:108409-83-2
- [D-Phe12]-Bombesin
Catalog No.:BCC5844
CAS No.:108437-87-2
- [D-Phe12,Leu14]-Bombesin
Catalog No.:BCC6020
CAS No.:108437-88-3
- Ilexsaponin A
Catalog No.:BCN7867
CAS No.:108524-93-2
- Ilexgenin A
Catalog No.:BCC9233
CAS No.:108524-94-3
- Eupahualin C
Catalog No.:BCN7234
CAS No.:108525-39-9
- 23S-hydroxy-11,15-dioxo-ganoderic acid DM
Catalog No.:BCN8131
CAS No.:1085273-49-9
- Pyridostatin
Catalog No.:BCC1875
CAS No.:1085412-37-8
- Lumichrome
Catalog No.:BCN7083
CAS No.:1086-80-2
-
4-Hydroxy-Teriflunomide
Catalog No.:BCC4734
CAS No.:
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
- Mizolastine
Catalog No.:BCC4521
CAS No.:108612-45-9
Further reactions of t-butyl 3-oxobutanthioate and t-butyl 4-diethyl-phosphono-3-oxobutanthioate : Carbonyl coupling reactions, amination, use in the preparation of 3-acyltetramic acids and application to the total synthesis of fuligorubin A.
Tetrahedron Volume 48, Issue 6, 1992, Pages 1145–1174
The use of t-butyl-3-oxobutanthioate (1) and t-butyl 4-diethylphosphono-3-oxobutanthioate (2) for the preparation of homologated derivatives suitable for amination in the presence of silver (I) trifluoroacetate to afford the corresponding β-ketoamides is discussed. In particular Wadsworth-Emmons coupling reactions of (2) with various carbonyl compounds gave good yields of E-substituted products. Many of the β-ketoamides were shown to be suitable precursors for 3-acyltetramic acids using a Dieckmann cyclisation with tetra-n-butyl ammonium fluoride as the cyclising base. These new reactions were applied to the total synthesis of the polyene 3-acyltetramic acid Fuligorubin A.


