GW5074C-Raf inhibitor,potent and selective CAS# 220904-83-6 |
- Rosiglitazone maleate
Catalog No.:BCC2262
CAS No.:155141-29-0
- Gemfibrozil
Catalog No.:BCC4783
CAS No.:25812-30-0
- Rosiglitazone HCl
Catalog No.:BCC2269
CAS No.:302543-62-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 220904-83-6 | SDF | Download SDF |
| PubChem ID | 5924208 | Appearance | Powder |
| Formula | C15H8Br2INO2 | M.Wt | 520.95 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (191.96 mM) *"≥" means soluble, but saturation unknown. | ||
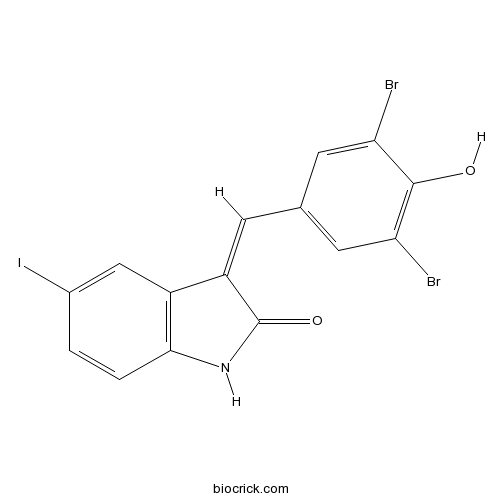
| Chemical Name | (3Z)-3-[(3,5-dibromo-4-hydroxyphenyl)methylidene]-5-iodo-1H-indol-2-one | ||
| SMILES | C1=CC2=C(C=C1I)C(=CC3=CC(=C(C(=C3)Br)O)Br)C(=O)N2 | ||
| Standard InChIKey | LMXYVLFTZRPNRV-KMKOMSMNSA-N | ||
| Standard InChI | InChI=1S/C15H8Br2INO2/c16-11-4-7(5-12(17)14(11)20)3-10-9-6-8(18)1-2-13(9)19-15(10)21/h1-6,20H,(H,19,21)/b10-3- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective and cell-permeable c-Raf1 kinase inhibitor (IC50 = 9 nM). Displays ≥ 100-fold selectivity for raf kinase over CDK1, CDK2, c-src, ERK2, MEK, p38, Tie2, VEGFR2 and c-fm. |

GW5074 Dilution Calculator

GW5074 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9196 mL | 9.5979 mL | 19.1957 mL | 38.3914 mL | 47.9893 mL |
| 5 mM | 0.3839 mL | 1.9196 mL | 3.8391 mL | 7.6783 mL | 9.5979 mL |
| 10 mM | 0.192 mL | 0.9598 mL | 1.9196 mL | 3.8391 mL | 4.7989 mL |
| 50 mM | 0.0384 mL | 0.192 mL | 0.3839 mL | 0.7678 mL | 0.9598 mL |
| 100 mM | 0.0192 mL | 0.096 mL | 0.192 mL | 0.3839 mL | 0.4799 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GW5074 is a synthetic inhibitor of c-Raf with IC50 value of 9nM [1].
GW5074 is found to be a potent inhibitor of c-Raf and has no effect on cdk1, cdk2, c-src, p38 MAP kinase, VEGFR2 and c-fms. GW5074 also shows no direct effect on JNK proteins and p38 MAP kinase. In neurons, GW5074 reduces the phosphorylation level of c-Raf at Ser259, results in an induction of c-Raf activity. It is further proved that GW5074 shows neuroprotective efficacy when its dose is below 1μM. In addition, GW5074 suppresses cell apoptosis through delaying the down-regulation of Akt phosphorylation and preventing the activation of GSK3β. In the in vivo model of Huntington’s disease, GW5074 protects neurons via resisting 3-NP-induced striatal neurodegeneration [1].
References:
[1] Burgess S, Echeverria V. Raf inhibitors as therapeutic agents against neurodegenerative diseases. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 2010, 9(1): 120-127.
- 2,3-O-Isopropylidenyl euscaphic acid
Catalog No.:BCN4944
CAS No.:220880-90-0
- Thunalbene
Catalog No.:BCN3688
CAS No.:220862-05-5
- 7-Methoxy-8-Hydroxy-4-Methylcoumarin
Catalog No.:BCC8291
CAS No.:22084-94-2
- NG,NG-Dimethylarginine dihydrochloride
Catalog No.:BCN1485
CAS No.:220805-22-1
- Aminopurvalanol A
Catalog No.:BCC7249
CAS No.:220792-57-4
- Aquifoliunine E-III
Catalog No.:BCN3096
CAS No.:220751-20-2
- Curlignan
Catalog No.:BCN3977
CAS No.:220736-54-9
- Ketoprofen
Catalog No.:BCC4430
CAS No.:22071-15-4
- Oxonic acid potassium salt
Catalog No.:BCC4165
CAS No.:2207-75-2
- Antisauvagine-30
Catalog No.:BCC5868
CAS No.:220673-95-0
- RJR-2403 oxalate
Catalog No.:BCC1902
CAS No.:220662-95-3
- Tigecycline
Catalog No.:BCC2499
CAS No.:220620-09-7
- 13-O-Cinnamoylbaccatin III
Catalog No.:BCN7344
CAS No.:220932-65-0
- 13-O-Deacetyltaxumairol Z
Catalog No.:BCN4945
CAS No.:220935-39-7
- Antalarmin hydrochloride
Catalog No.:BCC7480
CAS No.:220953-69-5
- Lumiracoxib
Catalog No.:BCC4440
CAS No.:220991-20-8
- Guaiacol glycidyl ether
Catalog No.:BCC8992
CAS No.:2210-74-4
- 1''-Methoxyerythrinin C
Catalog No.:BCN3966
CAS No.:221002-11-5
- 1alpha,4beta,10beta-Trihydroxyguaia-2,11(13)-dien-12,6alpha-olide
Catalog No.:BCN7483
CAS No.:221148-94-3
- Erysubin A
Catalog No.:BCN4946
CAS No.:221150-18-1
- Erysubin B
Catalog No.:BCN4947
CAS No.:221150-19-2
- Glyoxalase I inhibitor
Catalog No.:BCC1598
CAS No.:221174-33-0
- Z-Lys-OH
Catalog No.:BCC2764
CAS No.:2212-75-1
- Z-Lys(Boc)-OH.DCHA
Catalog No.:BCC2591
CAS No.:2212-76-2
Raf-kinase inhibitor GW5074 shows antibacterial activity against methicillin-resistant Staphylococcus aureus and potentiates the activity of gentamicin.[Pubmed:27652456]
Future Med Chem. 2016 Oct;8(16):1941-1952.
AIM: Increasing antimicrobial resistance has compromised the effectiveness of many antibiotics, including those used to treat staphylococcal infections like methicillin-resistant Staphylococcus aureus. The development of combination therapies, where antimicrobial agents are used with compounds that inhibit resistance pathways is a promising strategy. Results/methodology: The Raf kinase inhibitor GW5074 exhibited selective in vitro activity against Gram-positive bacteria, including clinical isolates of S. aureus with a minimum inhibitory concentration (MIC) of 2-8 microg/ml. GW5074 was effective in vivo in the Galleria mellonella infection model. The compound showed synergy with gentamicin by lowering MIC by fourfold, compared with gentamicin MIC alone. CONCLUSION: This work demonstrates the antimicrobial properties of GW5074 and supports further investigation of the kinase inhibitors as antibiotic adjuvants.
Inhibition of the human deacylase Sirtuin 5 by the indole GW5074.[Pubmed:23195732]
Bioorg Med Chem Lett. 2013 Jan 1;23(1):143-6.
Sirtuins are NAD(+) consuming protein deacylases involved in many cellular processes from DNA-repair to metabolism. Their contribution to age-related and metabolic diseases makes them attractive pharmaceutical targets. Few pharmacological inhibitors have been reported yet for human Sirt5 since substrates and assays for reliable testing of its activity were unavailable until recently, and most modulators of other Sirtuins were not tested against Sirt5 and therefore have only partially characterized isoform selectivities. We used here improved substrates and assays for testing of known Sirtuin inhibitors for their effects on two activities of human Sirt5, the generic Sirtuin activity deacetylation and the more pronounced Sirt5 activity desuccinylation. Our tests show that most of the compounds have no significant effect on either Sirt5 activity. The indole GW5074, however, was found to be a potent inhibitor for Sirt5's desuccinylation activity, identifying a first pharmacological scaffold for development into Sirt5-specific inhibitors. Interestingly, the compound showed weaker effects in Sirt5 deacetylation assays and also varying potencies against different peptide sequences, indicating a substrate-specific effect of GW5074.
GW5074 and PP2 kinase inhibitors implicate nontraditional c-Raf and Lyn function as drivers of retinoic acid-induced maturation.[Pubmed:25817574]
Cell Signal. 2015 Aug;27(8):1666-75.
The multivariate nature of cancer necessitates multi-targeted therapy, and kinase inhibitors account for a vast majority of approved cancer therapeutics. While acute promyelocytic leukemia (APL) patients are highly responsive to retinoic acid (RA) therapy, kinase inhibitors have been gaining momentum as co-treatments with RA for non-APL acute myeloid leukemia (AML) differentiation therapies, especially as a means to treat relapsed or refractory AML patients. In this study GW5074 (a c-Raf inhibitor) and PP2 (a Src-family kinase inhibitor) enhanced RA-induced maturation of t(15;17)-negative myeloblastic leukemia cells and rescued response in RA-resistant cells. PD98059 (a MEK inhibitor) and Akti-1/2 (an Akt inhibitor) were less effective, but did tend to promote maturation-uncoupled G1/G0 arrest, while wortmannin (a PI3K inhibitor) did not enhance differentiation surface marker expression or growth arrest. PD98059 and Akti-1/2 did not enhance differentiation markers and have potential, antagonistic off-targets effects on the aryl hydrocarbon receptor (AhR), but neither could the AhR agonist 6-formylindolo(3,2-b)carbazole (FICZ) rescue differentiation events in the RA-resistant cells. GW5074 rescued early CD38 expression in RA-resistant cells exhibiting an early block in differentiation before CD38 expression, while for RA-resistant cells with differentiation blocked later, PP2 rescued the later differentiation marker CD11b; but surprisingly, the combination of the two was not synergistic. Kinases c-Raf, Src-family kinases Lyn and Fgr, and PI3K display highly correlated signaling changes during RA treatment, while activation of traditional downstream targets (Akt, MEK/ERK), and even the surface marker CD38, were poorly correlated with c-Raf or Lyn during differentiation. This suggests that an interrelated kinase module involving c-Raf, PI3K, Lyn and perhaps Fgr functions in a nontraditional way during RA-induced maturation or during rescue of RA induction therapy using inhibitor co-treatment in RA-resistant leukemia cells.
The Raf-1 inhibitor GW5074 and the ERK1/2 pathway inhibitor U0126 ameliorate PC12 cells apoptosis induced by 6-hydroxydopamine.[Pubmed:22957439]
Pharmazie. 2012 Aug;67(8):718-24.
6-Hydroxydopamine (6-OHDA) is a widely used dopaminergic neurotoxin that leads to cell apoptosis in vivo and in vitro, and is a widely accepted experimental model of neurodegeneration in Parkinson's disease. However, the molecular mechanisms responsible for 6-OHDA-induced cell apoptosis are unclear. We found that the treatment of PC12 cells with 6-OHDA resulted in a significant decrease in cell viability and elevated apoptosis as detected by MTT assay, Hoechst 33258 staining, and flow cytometry. In addition, 6-OHDA induced a time-dependent phosphorylation of ERK1/2 at Thr-202/Tyr-204 and of Raf-1 at Ser-338, but a decreased level of Raf-1 phosphorylation at Ser-259. Phosphorylation of ERK1/2 at Thr-202/Tyr-204 and Raf-1 at Ser-338 were inhibited by the Raf-1 inhibitor GW5074, while the ERK1/2 pathway inhibitor U0126 decreased phosphorylation of ERK1/2. Furthermore, 6-OHDA-induced PC12 cells apoptosis was suppressed by GW5074 and U0126. Our results suggest that GW5074 and U0126 act as neuroprotants against 6-OHDA toxicity in PC12 cells by modulating Raf-1/ERK1/2 signaling systems.
Inhibition of ATF-3 expression by B-Raf mediates the neuroprotective action of GW5074.[Pubmed:18194435]
J Neurochem. 2008 May;105(4):1300-12.
GW5074 a brain-permeable 3' substituted indolone, protects neurons from death in culture and in an in vivo paradigm of neurodegeneration. Using low potassium (LK) induced apoptosis of cerebellar granule neurons, we report here that the protective action of GW5074 is mediated through the activation of B-Raf. Over-expression of a kinase-dead form of B-Raf blocks the ability of GW5074 to neuroprotect, whereas over-expression of active forms of B-Raf protect even in the absence of GW5074. Although mitogen-activated protein kinase kinase (MEK) and extracellular signal-regulated protein kinase (ERK) are activated by GW5074, pharmacological inhibition of MEK-ERK signaling by U0126 or PD98059 does not reduce neuroprotection suggesting that B-Raf signals through a non-canonical signaling pathway. GeneChip microarray analyses identified activating transcription factor-3 (ATF-3) as a gene whose expression is induced by LK but that is negatively regulated by GW5074. Forced inhibition of ATF-3 expression using siRNA protects neurons against LK-induced apoptosis, whereas the over-expression of ATF-3 blocks GW5074-mediated neuroprotection. Not unexpectedly, expression of active B-Raf inhibits the apoptosis-associated increase in ATF-3 expression. We extended our work to include three other 3' substituted indolones - a commercially available inhibitor of RNA-dependent protein kinase and two novel compounds designated as SK4 and SK6. Like GW5074, RNA-dependent protein kinase inhibitor, SK4, and SK6 all inhibited c-Raf in vitro but activated B-Raf in neuronal cultures. All four compounds also inhibited ATF-3 expression. Taken together our results indicate that all four indolones mediate neuroprotection by a common mechanism which involves B-Raf activation, and that a downstream target of B-Raf is ATF-3.
VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide.[Pubmed:12388327]
Am J Physiol Heart Circ Physiol. 2003 Jan;284(1):H92-H100.
We tested the hypothesis that VEGF regulates endothelial hyperpermeability to macromolecules by activating the ERK-1/2 MAPK pathway. We also tested whether PKC and nitric oxide (NO) mediate VEGF-induced increases in permeability via the ERK-1/2 pathway. FITC-Dextran 70 flux across human umbilical vein endothelial cell monolayers served as an index of permeability, whereas Western blots assessed the phosphorylation of ERK-1/2. VEGF-induced hyperpermeability was inhibited by antisense DNA oligonucleotides directed against ERK-1/2 and by blockade of MEK and Raf-1 activities (20 microM PD-98059 and 5 microM GW-5074). These blocking agents also reduced ERK-1/2 phosphorylation. The PKC inhibitor bisindolylmaleimide I (10 microM) blocked both VEGF-induced ERK-1/2 activation and hyperpermeability. The NO synthase (NOS) inhibitor N(G)-nitro-l-arginine methyl ester (200 microM) and the NO scavenger 2-phenyl-4,4,5,5-tetramethylimidiazoline-1-oxyl-3-oxide (100 microM) abolished VEGF-induced hyperpermeability but did not block ERK-1/2 phosphorylation. These observations demonstrate VEGF-induced hyperpermeability involves activation of PKC and NOS as well as Raf-1, MEK, and ERK-1/2. Furthermore, our data suggest that ERK-1/2 and NOS are elements of different signaling pathways in VEGF-induced hyperpermeability.
Involvement of Raf-1 in chronic delta-opioid receptor agonist-mediated adenylyl cyclase superactivation.[Pubmed:12223234]
Eur J Pharmacol. 2002 Sep 6;451(1):101-2.
Chronic delta-opioid receptor agonist treatment of Chinese hamster ovary (CHO) cells stably expressing the human delta-opioid receptor (hDOR/CHO) leads to increased cAMP formation after the removal of the agonist (adenylyl cyclase superactivation). We have previously found that at the same time, chronic delta-opioid receptor agonist treatment augments phosphorylation of the adenylyl cyclase VI isoenzyme. Since phosphorylation of adenylyl cyclase VI by Raf-1 protein kinase was recently shown, we tested the role of Raf-1 in adenylyl cyclase superactivation in hDOR/CHO cells. We found that pretreatment of the cells with the selective Raf-1 inhibitor GW5074 (3-(3,5-dibromo-4-hydroxybenzylidene-5-iodo-1,3-dihydro-indol-2-one) (10 microM, 30 min) attenuates chronic deltorphin II-mediated increase in forskolin-stimulated cAMP formation by 40% (n = 6, P < 0.05). Better understanding of the molecular mechanism of adenylyl cyclase superactivation should aid in the development of analgesics that act longer and have fewer side effects.
The discovery of potent cRaf1 kinase inhibitors.[Pubmed:10698440]
Bioorg Med Chem Lett. 2000 Feb 7;10(3):223-6.
A series of benzylidene-1H-indol-2-one (oxindole) derivatives was synthesized and evaluated as cRaf-1 kinase inhibitors. The key features of the molecules were the donor/acceptor motif common to kinase inhibitors and a critical acidic phenol flanked by two substitutions. Diverse 5-position substitutions provided compounds with low nanomolar kinase enzyme inhibition and inhibited the intracellular MAPK pathway.


