Gallic acidCAS# 149-91-7 |
Quality Control & MSDS
Number of papers citing our products

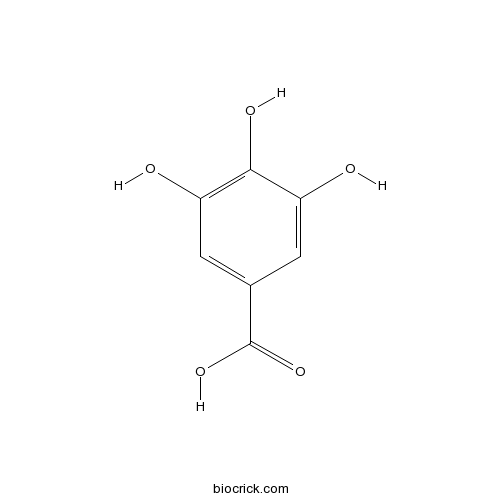
Chemical structure

3D structure
| Cas No. | 149-91-7 | SDF | Download SDF |
| PubChem ID | 370 | Appearance | White powder |
| Formula | C7H6O5 | M.Wt | 170.1 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | 3,4,5-Trihydroxybenzoic acid | ||
| Solubility | DMSO : 100 mg/mL (587.82 mM; Need ultrasonic and warming) | ||
| Chemical Name | 3,4,5-trihydroxybenzoic acid | ||
| SMILES | C1=C(C=C(C(=C1O)O)O)C(=O)O | ||
| Standard InChIKey | LNTHITQWFMADLM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H6O5/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1-2,8-10H,(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gallic acid, is a histone acetyltransferase inhibitor and a potent inhibitor of brush border sucrase and other disaccharidases, which has powerful antioxidant, anti-tumor, and anti-tyrosinase activities. It can potentially interfere with the digestive functions of the intestine. It can efficiently block neuronal cell death by downregulating the expression of cytokines and the in vivo levels of NF-κB acetylation, is a possible therapeutic approach for alleviating the inflammatory progression of Alzheimer disease. |
| Targets | NF-kB | Beta Amyloid | Tyrosinase |
| In vitro | Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid[Reference: WebLink]Food Chem., 2002, 79(3):307-13.The antioxidant and pro-oxidant properties of ascorbic acid (AA) and Gallic acid (GA) were investigated.
Antimelanogenic and antioxidant properties of gallic acid.[Pubmed: 17541153]Biol Pharm Bull. 2007 Jun;30(6):1052-5.To find novel skin-whitening agents, the melanogenesis inhibitory action of Gallic acid (GA) was investigated.
|
| In vivo | Gallic acid inhibits brush border disaccharidases in mammalian intestine.[Reference: WebLink]Nut. Res., 2007, 27(4):230-5.Intestinal epithelium constitutes the primary target tissue for interaction with dietary micronutrients. The objective of this study was to determine if Gallic acid, a polyphenol that is an important constituent of various edible plant-based foods, affects brush border disaccharidases in mammalian intestine.
Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice.[Pubmed: 11707653]Anticancer Drugs. 2001 Nov;12(10):847-52.We previously reported that Gallic acid (3,4,5-trihydroxybenzoic acid), a naturally occurring plant phenol, can induce apoptosis in four kinds of human lung cancer cell lines in vitro. The present study further investigated the in vivo anti-tumor effects of orally administered Gallic acid.
|
| Cell Research | Radical intensity and cytotoxic activity of curcumin and gallic acid.[Pubmed: 9858929]Anticancer Res. 1998 Sep-Oct;18(5A):3487-91.Natural phenolic compounds, curcumin and Gallic acid, were compared for their cytotoxic activity in relation to their radical modulating activity.
|
| Animal Research | Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation.[Pubmed: 22038937]Mol Nutr Food Res. 2011 Dec;55(12):1798-808.We examined the biological effect of Gallic acid (GA) as a nuclear factor (NF)-κB acetyltransferase inhibitor on microglial-mediated β-amyloid neurotoxicity and restorative effects on the Aβ-induced cognitive dysfunction.
|

Gallic acid Dilution Calculator

Gallic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.8789 mL | 29.3945 mL | 58.7889 mL | 117.5779 mL | 146.9724 mL |
| 5 mM | 1.1758 mL | 5.8789 mL | 11.7578 mL | 23.5156 mL | 29.3945 mL |
| 10 mM | 0.5879 mL | 2.9394 mL | 5.8789 mL | 11.7578 mL | 14.6972 mL |
| 50 mM | 0.1176 mL | 0.5879 mL | 1.1758 mL | 2.3516 mL | 2.9394 mL |
| 100 mM | 0.0588 mL | 0.2939 mL | 0.5879 mL | 1.1758 mL | 1.4697 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Scopolamine butylbromide
Catalog No.:BCN5006
CAS No.:149-64-4
- Erythritol
Catalog No.:BCN1664
CAS No.:149-32-6
- Fmoc-Inp-OH
Catalog No.:BCC3266
CAS No.:148928-15-8
- 3-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl-2-propenal
Catalog No.:BCC8600
CAS No.:148901-68-2
- Fmoc-L-Arg(Aloc)2-OH
Catalog No.:BCC2564
CAS No.:148893-34-9
- HATU
Catalog No.:BCC2813
CAS No.:148893-10-1
- YM 511
Catalog No.:BCC6002
CAS No.:148869-05-0
- Ivabradine HCl
Catalog No.:BCC4350
CAS No.:148849-67-6
- Rutamarin
Catalog No.:BCN7509
CAS No.:14882-94-1
- Bismuth Subsalicylate
Catalog No.:BCC3739
CAS No.:14882-18-9
- Carboxy-PTIO, potassium salt
Catalog No.:BCC6789
CAS No.:148819-94-7
- (R)-2-Methylcysteine HCl
Catalog No.:BCC4017
CAS No.:148766-37-4
- D-Isomenthol
Catalog No.:BCN8540
CAS No.:23283-97-8
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- (S)-WAY 100135 dihydrochloride
Catalog No.:BCC6993
CAS No.:149007-54-5
- H-D-Trp-OMe.HCl
Catalog No.:BCC3118
CAS No.:14907-27-8
- Brusatol
Catalog No.:BCN8278
CAS No.:14907-98-3
- AG 825
Catalog No.:BCC7113
CAS No.:149092-50-2
- Brugine
Catalog No.:BCN1899
CAS No.:14912-30-2
- Cratoxylone
Catalog No.:BCN3875
CAS No.:149155-01-1
- Homaloside D
Catalog No.:BCN1661
CAS No.:149155-19-1
- Eriodictyol chalcone
Catalog No.:BCN8276
CAS No.:14917-41-0
- 3-(2,4-Dihydroxybenzyl)-5-hydroxy-7,8-dimethoxy-6-methylchroman-4-one
Catalog No.:BCN6634
CAS No.:149180-48-3
- Irenolone
Catalog No.:BCN7146
CAS No.:149184-19-0
Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice.[Pubmed:11707653]
Anticancer Drugs. 2001 Nov;12(10):847-52.
We previously reported that Gallic acid (3,4,5-trihydroxybenzoic acid), a naturally occurring plant phenol, can induce apoptosis in four kinds of human lung cancer cell lines in vitro. The present study further investigated the in vivo anti-tumor effects of orally administered Gallic acid. Gallic acid reduced cell viability of LL-2 mouse lung cancer cells in vitro dose dependently, with a 50% inhibitory concentration (IC50) value of around 200 microM. C57Black mice were transplanted with LL-2 cells, and administered Gallic acid (1 mg/ml in drinking water, ad libitum) and/or cisplatin (4 mg/kg i.p. injection, once a week). The average weight of the transplanted tumors, obtained at 29 days after transplantation, in the mice of control, Gallic acid-treated cisplatin-treated and cisplatin plus Gallic acid-treated groups was 4.02, 3.65, 3.19 and 1.72 g, respectively. The average tumor weight of the mice treated with cisplatin combined with Gallic acid was significantly smaller than that of the control group (p<0.05). The amount of apoptotic cells in the tumor tissues of mice treated with Gallic acid and/or cisplatin was significantly higher than those of the control mice. Combination of Gallic acid and cisplatin increased the tumor cell apoptosis compared with the treatment with cisplatin alone. The present findings suggest that the combination of Gallic acid with an anti-cancer drug, including cisplatin, may be an effective protocol for lung cancer therapy.
Gallic acid, a histone acetyltransferase inhibitor, suppresses beta-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation.[Pubmed:22038937]
Mol Nutr Food Res. 2011 Dec;55(12):1798-808.
SCOPE: We examined the biological effect of Gallic acid (GA) as a nuclear factor (NF)-kappaB acetyltransferase inhibitor on microglial-mediated beta-amyloid neurotoxicity and restorative effects on the Abeta-induced cognitive dysfunction. METHODS AND RESULTS: The protective effects of GA on the survival of neuronal cells were assessed with an MTT assay and a co-culture system. For the co-culture experiments, both BV-2 and primary microglia cells were treated with GA prior to Abeta stimulation, and conditioned media were transferred to Neuro-2A cells. The mRNA and protein levels of inflammatory cytokines in both microglia and Neuro-2A cells were assessed with real-time polymerase chain reaction and western blotting. Inhibition of nuclear factor kappa B (NF-kappaB) acetylation with GA treatment resulted in reduced cytokine production in microglia cells and protection of neuronal cells from Abeta-induced neurotoxicity. Furthermore, we observed a restorative effect of GA on Abeta-induced cognitive dysfunction in mice with Y-maze and passive avoidance tests. Finally, we found that GA treatment efficiently blocked neuronal cell death by downregulating the expression of cytokines and the in vivo levels of NF-kappaB acetylation. CONCLUSION: These results suggest that selective inhibition of NF-kappaB acetylation by the histone acetyltransferase inhibitor GA is a possible therapeutic approach for alleviating the inflammatory progression of Alzheimer disease.
Radical intensity and cytotoxic activity of curcumin and gallic acid.[Pubmed:9858929]
Anticancer Res. 1998 Sep-Oct;18(5A):3487-91.
Natural phenolic compounds, curcumin and Gallic acid, were compared for their cytotoxic activity in relation to their radical modulating activity. These two compounds induced apoptotic cell death in human promyelocytic leukemic HL-60 cells and human oral squamous carcinoma HSC-4 cells. Curcumin was more cytotoxic than Gallic acid. Catalase reduced significantly the cytotoxic activity of Gallic acid, but not that of curcumin. ESR spectroscopy demonstrated that curcumin produced radicals under alkaline conditions, scavenged the superoxide anion radical, and enhanced the radical intensity of sodium ascorbate at higher concentrations. As compared with curcumin, Gallic acid produced higher amounts of radicals and more efficiently scavenged the superoxide anion radical. Gallic acid reduced the radical intensity of sodium ascorbate, suggesting a possible interaction between these two compounds. These data suggest that curcumin and Gallic acid induce apoptosis by different mechanisms.
Antimelanogenic and antioxidant properties of gallic acid.[Pubmed:17541153]
Biol Pharm Bull. 2007 Jun;30(6):1052-5.
To find novel skin-whitening agents, the melanogenesis inhibitory action of Gallic acid (GA) was investigated. In this current study, the effects of GA on mushroom tyrosinase, tyrosinase inhibitory activity, and melanin content were assessed in B16 melanoma cells (B16 cells). Results indicated that GA has a strong antityrosinase activity (IC50=3.59x10(-6) M). Furthermore, data on murine tyrosinase activity and melanin biosynthesis revealed that GA effectively suppressed murine tyrosinase action and the amount of melanin. To investigate the relation between GA's inhibition of melanogenesis and antioxidant activity, the effect of GA on reactive species (RS) generation and the reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio in were determined in B16 cells. Results indicated that GA effectively down-regulated the RS generation and enhanced the GSH/GSSG ratio. Based on these results, I propose that GA exerts antimelanogenic activity coupled with antioxidant properties by suppressing RS generation and maintaining a higher GSH/GSSG ratio.


