Ganoderic acid C2CAS# 103773-62-2 |
Quality Control & MSDS
Number of papers citing our products

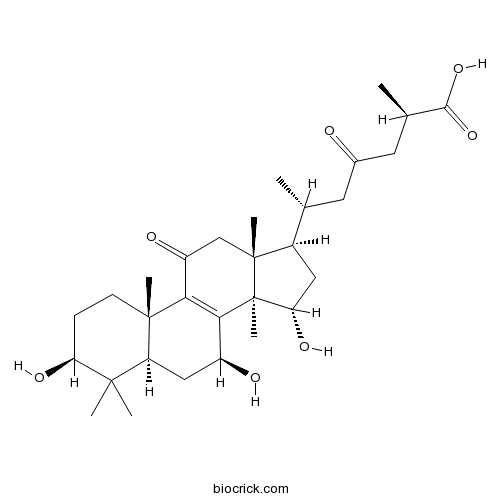
Chemical structure

3D structure
| Cas No. | 103773-62-2 | SDF | Download SDF |
| PubChem ID | 57396771 | Appearance | Powder |
| Formula | C30H46O7 | M.Wt | 518.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Lanost-8-en-26-oicacid;98296-48-1 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,6R)-2-methyl-4-oxo-6-[(3S,5R,7S,10S,13R,14R,15S,17R)-3,7,15-trihydroxy-4,4,10,13,14-pentamethyl-11-oxo-1,2,3,5,6,7,12,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]heptanoic acid | ||
| SMILES | CC(CC(=O)CC(C)C(=O)O)C1CC(C2(C1(CC(=O)C3=C2C(CC4C3(CCC(C4(C)C)O)C)O)C)C)O | ||
| Standard InChIKey | RERVSJVGWKIGTJ-RQLZKMEDSA-N | ||
| Standard InChI | InChI=1S/C30H46O7/c1-15(10-17(31)11-16(2)26(36)37)18-12-23(35)30(7)25-19(32)13-21-27(3,4)22(34)8-9-28(21,5)24(25)20(33)14-29(18,30)6/h15-16,18-19,21-23,32,34-35H,8-14H2,1-7H3,(H,36,37)/t15-,16-,18-,19+,21+,22+,23+,28+,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ganoderic acid C2 has anti-inflammatory,and anti-tumor-promoting activities. Ganoderic acid C2 can inhibit histamine release, it also has inhibitory effects on the induction of Epstein-Barr Virus early antigen. |
| Targets | Histamine Receptor | Immunology & Inflammation related |
| In vitro | Structural characterization of minor metabolites and pharmacokinetics of ganoderic acid C2 in rat plasma by HPLC coupled with electrospray ionization tandem mass spectrometry.[Pubmed: 23312386]J Pharm Biomed Anal. 2013 Mar 5;75:64-73.
|
| Kinase Assay | Inhibition of aldose reductase in vitro by constituents of Ganoderma lucidum.[Pubmed: 20379959 ]Planta Med. 2010 Oct;76(15):1691-3.CHCl(3) extract of the fruiting body of Ganoderma lucidum was found to show inhibitory activity on human aldose reductase in vitro. From the acidic fraction, potent human aldose reductase inhibitors, Ganoderic acid C2 (1) and ganoderenic acid A (2), were isolated together with three related compounds. It was found that the free carboxyl group of Ganoderic acid C2 and ganoderenic acid A is essential in eliciting the inhibitory activity considering the much lower activity of their methyl esters. |
| Structure Identification | Bioorg Med Chem Lett. 2011 Dec 15;21(24):7295-7.Structure-activity relationships of ganoderma acids from Ganoderma lucidum as aldose reductase inhibitors.[Pubmed: 22047696]A series of lanostane-type triterpenoids, known as ganoderma acids were isolated from the fruiting body of Ganoderma lucidum. Some of these compounds were identified as active inhibitors of the in vitro human recombinant aldose reductase. To clarify the structural requirement for inhibition, some structure-activity relationships were determined. |

Ganoderic acid C2 Dilution Calculator

Ganoderic acid C2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9279 mL | 9.6395 mL | 19.279 mL | 38.5579 mL | 48.1974 mL |
| 5 mM | 0.3856 mL | 1.9279 mL | 3.8558 mL | 7.7116 mL | 9.6395 mL |
| 10 mM | 0.1928 mL | 0.9639 mL | 1.9279 mL | 3.8558 mL | 4.8197 mL |
| 50 mM | 0.0386 mL | 0.1928 mL | 0.3856 mL | 0.7712 mL | 0.9639 mL |
| 100 mM | 0.0193 mL | 0.0964 mL | 0.1928 mL | 0.3856 mL | 0.482 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
- R428
Catalog No.:BCC3692
CAS No.:1037624-75-1
- Shionone
Catalog No.:BCN1274
CAS No.:10376-48-4
- Fasudil
Catalog No.:BCC5262
CAS No.:103745-39-7
- Rehmaglutin D
Catalog No.:BCN5851
CAS No.:103744-84-9
- YK-4-279
Catalog No.:BCC2065
CAS No.:1037184-44-3
- Dihydrobonducellin
Catalog No.:BCN3731
CAS No.:103680-87-1
- Mirificin
Catalog No.:BCN2783
CAS No.:103654-50-8
- 2-Carbamoyl-3-hydroxy-1,4-naphthoquinone
Catalog No.:BCC8567
CAS No.:103646-20-4
- Ondansetron hydrochloride dihydrate
Catalog No.:BCC4213
CAS No.:103639-04-9
- Catechin 3-rhamnoside
Catalog No.:BCN5850
CAS No.:103630-03-1
- Cnidimol A
Catalog No.:BCN7167
CAS No.:103629-80-7
- MBX-2982
Catalog No.:BCC1732
CAS No.:1037792-44-1
- KX2-391 dihydrochloride
Catalog No.:BCC1686
CAS No.:1038395-65-1
- 7-Prenylumbelliferone
Catalog No.:BCN2938
CAS No.:10387-50-5
- 4'-O-Methyllicoflavanone
Catalog No.:BCN4827
CAS No.:1038753-13-7
- Lazabemide hydrochloride
Catalog No.:BCC7371
CAS No.:103878-83-7
- Cycloart-25-ene-3,24-diol
Catalog No.:BCN5852
CAS No.:10388-48-4
- Procyanidin A1
Catalog No.:BCN6809
CAS No.:103883-03-0
- Lacidipine
Catalog No.:BCC4403
CAS No.:103890-78-4
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- MK-4827 hydrochloride
Catalog No.:BCC4173
CAS No.:1038915-64-8
- MK-4827 tosylate
Catalog No.:BCC4174
CAS No.:1038915-73-9
- MK-4827 Racemate
Catalog No.:BCC5179
CAS No.:1038915-75-1
Structure-activity relationships of ganoderma acids from Ganoderma lucidum as aldose reductase inhibitors.[Pubmed:22047696]
Bioorg Med Chem Lett. 2011 Dec 15;21(24):7295-7.
A series of lanostane-type triterpenoids, known as ganoderma acids were isolated from the fruiting body of Ganoderma lucidum. Some of these compounds were identified as active inhibitors of the in vitro human recombinant aldose reductase. To clarify the structural requirement for inhibition, some structure-activity relationships were determined. Our structure-activity studies of ganoderma acids revealed that the OH substituent at C-11 is an important feature and the carboxylic group in the side chain is essential for the recognition of aldose reductase inhibitory activity. Moreover, double bond moiety at C-20 and C-22 in the side chain contributes to improving aldose reductase inhibitory activity. In the case of Ganoderic acid C2, all of OH substituent at C-3, C-7 and C-15 is important for potent aldose reductase inhibition. These results provide an approach to understanding the structural requirements of ganoderma acids from G. lucidum for aldose reductase inhibitor. This understanding is necessary to design a new-type of aldose reductase inhibitor.
Structural characterization of minor metabolites and pharmacokinetics of ganoderic acid C2 in rat plasma by HPLC coupled with electrospray ionization tandem mass spectrometry.[Pubmed:23312386]
J Pharm Biomed Anal. 2013 Mar 5;75:64-73.
The metabolites and pharmacokinetics of Ganoderic acid C2 (GAC2), a bioactive triterpenoid in Ganoderma lucidum in rat plasma were investigated by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS). Totally, ten minor phase I metabolites of GAC2 were characterized after oral administration of GAC2, on the basis of their mass fragmentation pathways or direct comparison with authentic compounds by high-performance liquid chromatography coupled with diode array detection and electrospray ion trap tandem mass spectrometry (HPLC-DAD-ESI-MS(n)), and liquid chromatography coupled with electrospray ionization hybrid ion trap and time-of-flight mass spectrometry (LC-ESI-IT-TOF/MS) methods. Moreover, a rapid and specific method for quantification of GAC2 in rat plasma after oral administration was developed by using a liquid-liquid extraction procedure and HPLC-ESI-MS/MS analysis. It is the first time to report the metabolites and pharmacokinetics of GAC2.


