HymenoxinCAS# 56003-01-1 |
Quality Control & MSDS
Number of papers citing our products

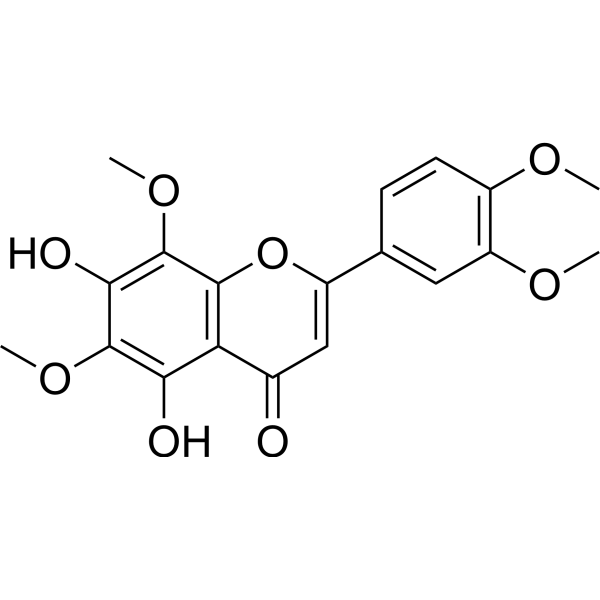
Chemical structure

| Cas No. | 56003-01-1 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C19H18O8 | M.Wt | 374.34 |
| Type of Compound | Flavones/Flavanones | Storage | Desiccate at -20°C |
| Synonyms | 5,7-Dihydroxy-6,8,3′,4′-tetramethoxyflavone | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hymenoxin Dilution Calculator

Hymenoxin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6714 mL | 13.3568 mL | 26.7137 mL | 53.4274 mL | 66.7842 mL |
| 5 mM | 0.5343 mL | 2.6714 mL | 5.3427 mL | 10.6855 mL | 13.3568 mL |
| 10 mM | 0.2671 mL | 1.3357 mL | 2.6714 mL | 5.3427 mL | 6.6784 mL |
| 50 mM | 0.0534 mL | 0.2671 mL | 0.5343 mL | 1.0685 mL | 1.3357 mL |
| 100 mM | 0.0267 mL | 0.1336 mL | 0.2671 mL | 0.5343 mL | 0.6678 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pebrellin
Catalog No.:BCX1881
CAS No.:13509-93-8
- Chrysindin A
Catalog No.:BCX1880
CAS No.:1374852-85-3
- 3-Epidigitoxigenin
Catalog No.:BCX1879
CAS No.:545-52-8
- Marsdenoside B
Catalog No.:BCX1878
CAS No.:858360-57-3
- 11α-O-Tigloyl-12β-O-acetyltenacigenin B
Catalog No.:BCX1877
CAS No.:154022-51-2
- Ingol-7,8,12-triacetate-3-phenylacetate
Catalog No.:BCX1876
CAS No.:944799-46-6
- Euphorblin R
Catalog No.:BCX1875
CAS No.:2230806-06-9
- Strictinin
Catalog No.:BCX1874
CAS No.:517-46-4
- Tenuiphenone B
Catalog No.:BCX1873
CAS No.:870298-00-3
- Glomeratide A
Catalog No.:BCX1872
CAS No.:1072028-74-0
- Machilin A
Catalog No.:BCX1871
CAS No.:110269-50-6
- Marsdenoside A
Catalog No.:BCX1870
CAS No.:858360-56-2
- Dehydrodanshenol A
Catalog No.:BCX1883
CAS No.:1444618-61-4
- 2,4,6-Trimethoxyl-3-methylacetophenone
Catalog No.:BCX1884
CAS No.:39701-13-8
- Sibiriquinone A
Catalog No.:BCX1885
CAS No.:723300-08-1
- 3-Epigitoxigenin
Catalog No.:BCX1886
CAS No.:465-10-1
- Lasiokaurinin
Catalog No.:BCX1887
CAS No.:52554-74-2
- Dichlorogelignate
Catalog No.:BCX1888
CAS No.:164030-91-5
- Digoxigenin
Catalog No.:BCX1889
CAS No.:1672-46-4
- 17α-Gitoxigenin
Catalog No.:BCX1890
CAS No.:4433-59-4
- Digitalin, 16-anhydro-
Catalog No.:BCX1891
CAS No.:7044-34-0
- Abrine D
Catalog No.:BCX1896
CAS No.:862504-05-0
- 4-((2-ethylidene-4-hydroxy-6-methylhept-5-en-1-yl)oxy)-bergaptol
Catalog No.:BCX1892
CAS No.:2519548-06-0
- 3,5,6,6-tetramethyl-4,5,5a,6,7,8-hexahydrocyclopenta[c]pentalen-2(1H)-one
Catalog No.:BCX1893
CAS No.:1689570-10-2
Identification of Bioactive Phytochemicals in Leaf Protein Concentrate of Jerusalem Artichoke (Helianthus tuberosus L.).[Pubmed:32674454]
Plants (Basel). 2020 Jul 14;9(7):889.
Jerusalem artichoke (JA) is widely known to have inulin-rich tubers. However, its fresh aerial biomass produces significant levels of leaf protein and economic bioactive phytochemicals. We have characterized leaf protein concentrate (JAPC) isolated from green biomass of three Jerusalem artichoke clones, Alba, Fuseau, and Kalevala, and its nutritional value for the human diet or animal feeding. The JAPC yield varied from 28.6 to 31.2 g DM kg(-1) green biomass with an average total protein content of 33.3% on a dry mass basis. The qualitative analysis of the phytochemical composition of JAPC was performed by ultra-high performance liquid chromatography-electrospray ionization-Orbitrap/mass spectrometry analysis (UHPLC-ESI-ORBITRAP-MS/MS). Fifty-three phytochemicals were successfully identified in JAPC. In addition to the phenolic acids (especially mono- and di-hydroxycinnamic acid esters of quinic acids) several medically important hydroxylated methoxyflavones, i.e., dimethoxy-tetrahydroxyflavone, dihydroxy-methoxyflavone, Hymenoxin, and nevadensin, were detected in the JAPC for the first time. Liquiritigenin, an estrogenic-like flavanone, was measured in the JAPC as well as butein and kukulkanin B, as chalcones. The results also showed high contents of the essential amino acids and polyunsaturated fatty acids (PUFAs; 66-68%) in JAPC. Linolenic acid represented 39-43% of the total lipid content; moreover, the ratio between omega-6 and omega-3 fatty acids in the JAPC was ~0.6:1. Comparing the JA clones, no major differences in phytochemicals, fatty acid, or amino acid compositions were observed. This paper confirms the economic and nutritional value of JAPC as it is not only an alternative plant protein source but also as a good source of biological valuable phytochemicals.
Antifungal and antibacterial activity and chemical composition of polar and non-polar extracts of Athrixia phylicoides determined using bioautography and HPLC.[Pubmed:24330447]
BMC Complement Altern Med. 2013 Dec 13;13:356.
BACKGROUND: Athrixia phylicoides DC. (Asteraceae) is used medicinally in South Africa to treat a plethora of ailments, including heart problems, diabetes, diarrhoea, sores and infected wounds. It is also prepared in the form of a tea (hot decoction) taken as a refreshing, pleasant-tasting beverage with commercialization potential. METHODS: Extracts of the dried ground aerial parts were prepared using organic solvents (diethyl ether, dichloromethane/methanol, ethyl acetate and ethanol) and water. These extracts were subjected to HPLC, TLC and bioautography analysis with the aim of linking a range of peaks visualized in HPLC chromatography profiles to antibacterial and antifungal activity of the same extracts. RESULTS: HPLC revealed a group of compounds extracted by more than one solvent. Compounds identified include inositol, caffeic acid, quercetin, kaempferol, apigenin, Hymenoxin and oleanolic acid. The organic extracts displayed similar TLC profiles, and bioautography indicated approximately five antibacterial compounds, but only two antifungal compounds in these extracts. Bioautography indicated that cold water extracted the least antimicrobial compounds. CONCLUSIONS: Several previously unknown compounds were identified in Athrixia phylicoides extracts, and bioautography indicated a number of antibacterial and antifungal compounds. There were notable differences in chemical composition and bioactivity between the organic and aqueous extracts. Further research is necessary to fully characterize the active components of the extracts.
A distinctive flavonoid chemistry for the anomalous genus Biebersteinia.[Pubmed:11198823]
Phytochemistry. 2001 Jan;56(1):87-91.
Leaf surface extracts of Biebersteinia orphanidis have yielded a complex mixture of five flavones with the unusual 5,7-dihydroxy-6,8-dimethoxy A ring substitution pattern. They are acerosin, Hymenoxin, nevadensin, sudachitin and 5,7,4'-trihydroxy-6,8-dimethoxyflavone. Also present at the leaf surface are gardenin B, luteolin, apigenin, acacetin and the coumarin umbelliferone. The internal leaf flavonoids include the 7-glucosides of apigenin, luteolin and tricetin, together with the 7-rutinosides of apigenin and luteolin. This profile differs from those of B. heterostemon and B. odora. It appears that B. orphanidis is as highly distinctive in its flavonoid pattern as it is phytogeographically. The data also confirm the conclusion of other studies, including rbcL and atpB gene sequence analysis, that Biebersteinia is completely unrelated to the Geraniaceae, where it was once placed.
Structure of the flavone hymenoxin.[Pubmed:2025405]
Acta Crystallogr C. 1991 Feb 15;47 ( Pt 2):459-61.
2-(3,4-Dimethoxyphenyl)-5,7-dihydroxy-6,8-dimethoxy-4H-chromen-4-o ne, C19H18O8, Mr = 374.38, monoclinic, P2(1)/n, a = 9.026(4), b = 15.054(6), c = 12.829(6) A, beta = 100.98(4) degrees, V = 1711(1) A3, Z = 4, Dx = 1.450 g cm-3, lambda (Mo K alpha) = 0.71073 A, mu = 1.07 cm-1, F(000) = 784, T = 295 K, R = 0.0778 for 2280 independent reflections. The nearly planar AB ring system (0.04 A r.m.s.d.), O(1) to C(10), and the planar C ring (0.001 A r.m.s.d.) are almost coplanar with an interplanar angle of only 4.4(4) degrees. The methyl groups at C(6) and C(8) are rotated out of the molecular plane on opposite sides with torsion angles C(5)C(6)O(6)C(11) = -94.5(4) and C(7)C(8)O(8)C(12) = 109.0(4) degrees. The methyl groups of ring C are coplanar with the ring, C(3')C(4')O(4')"(13) = 0.2(5) and C(6')C(5')O(5')C(14) = 1.3(5) degrees. The carbonyl group forms an intramolecular hydrogen bond with O(5), O(5)...O(4) = 2.612(5), H(5O)...O(4) = 1.85(4) A, O(5)-H(5O)...O(4) = 151.9(8) degrees, and an intermolecular hydrogen bond with O(7) of an adjacent molecule, O(7)...O(4) (-0.5 + x, -0.5 - y, 0.5 + z) = 2.689(5), H(7O)...O(4) = 1.92(4) A, and O(7)-H(7O)...O(4) = 163.2(8) degrees.


