Imetit dihydrobromideStandard H3 and H4 agonist CAS# 32385-58-3 |
- DCC-2618
Catalog No.:BCC1520
CAS No.:1225278-16-9
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Motesanib
Catalog No.:BCC1776
CAS No.:453562-69-1
- OSI-930
Catalog No.:BCC1253
CAS No.:728033-96-3
- Masitinib (AB1010)
Catalog No.:BCC1260
CAS No.:790299-79-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
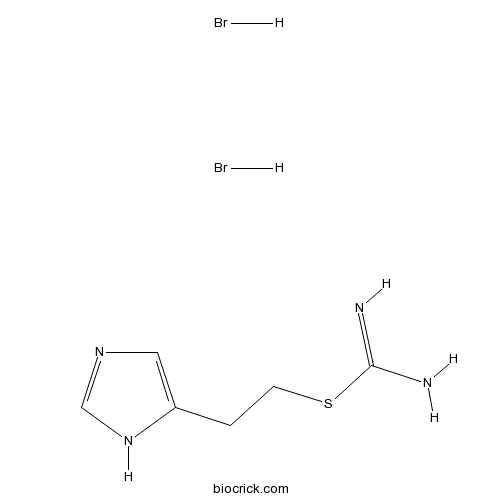
| Cas No. | 32385-58-3 | SDF | Download SDF |
| PubChem ID | 11957573 | Appearance | Powder |
| Formula | C6H12Br2N4S | M.Wt | 332.06 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | 2-(1H-imidazol-5-yl)ethyl carbamimidothioate;dihydrobromide | ||
| SMILES | C1=C(NC=N1)CCSC(=N)N.Br.Br | ||
| Standard InChIKey | DOBOYMKCRRLTRF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H10N4S.2BrH/c7-6(8)11-2-1-5-3-9-4-10-5;;/h3-4H,1-2H2,(H3,7,8)(H,9,10);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | An extremely potent, high affinity agonist at H3 and H4 receptors (Ki values are 0.3 and 2.7 nM respectively). Induces shape change in eosinophils with an EC50 of 25 nM. Centrally active following systemic administration. Also available as part of the Histamine H3 Receptor. |

Imetit dihydrobromide Dilution Calculator

Imetit dihydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0115 mL | 15.0575 mL | 30.115 mL | 60.2301 mL | 75.2876 mL |
| 5 mM | 0.6023 mL | 3.0115 mL | 6.023 mL | 12.046 mL | 15.0575 mL |
| 10 mM | 0.3012 mL | 1.5058 mL | 3.0115 mL | 6.023 mL | 7.5288 mL |
| 50 mM | 0.0602 mL | 0.3012 mL | 0.6023 mL | 1.2046 mL | 1.5058 mL |
| 100 mM | 0.0301 mL | 0.1506 mL | 0.3012 mL | 0.6023 mL | 0.7529 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Medicarpin
Catalog No.:BCN5241
CAS No.:32383-76-9
- 9,9-Bis(4-hydroxyphenyl)fluorene
Catalog No.:BCC8795
CAS No.:3236-71-3
- 4-Nitrobenzyl carbamate
Catalog No.:BCN3286
CAS No.:32339-07-4
- p-Cresyl sulfate
Catalog No.:BCC4013
CAS No.:3233-58-7
- SCS
Catalog No.:BCC7266
CAS No.:3232-36-8
- Triphosgene
Catalog No.:BCC2848
CAS No.:32315-10-9
- H-Threoninol
Catalog No.:BCC2705
CAS No.:3228-51-1
- 4-Methoxy-1-methylquinolin-2-one
Catalog No.:BCN4824
CAS No.:32262-18-3
- Methyl 3-cyclopropyl-3-oxopropionate
Catalog No.:BCC9038
CAS No.:32249-35-7
- SCH 221510
Catalog No.:BCC7612
CAS No.:322473-89-2
- 2alpha,7beta,13alpha-Triacetoxy-5alpha-cinnamoyloxy-9beta-hydroxy-2(3->20)abeotaxa-4(20),11-dien-10-one
Catalog No.:BCN7208
CAS No.:322471-42-1
- Calcitriol
Catalog No.:BCC4950
CAS No.:32222-06-3
- Dexfenfluramine hydrochloride
Catalog No.:BCC5927
CAS No.:3239-45-0
- AKTide-2T
Catalog No.:BCC5908
CAS No.:324029-01-8
- Vitexin-4'-Rhamnoside(Rg)
Catalog No.:BCC8369
CAS No.:32426-34-9
- Isofebrifugine
Catalog No.:BCN3270
CAS No.:32434-44-9
- H-D-Cys-OH.H2O.HCl
Catalog No.:BCC2913
CAS No.:32443-99-5
- Khasianine
Catalog No.:BCN2530
CAS No.:32449-98-2
- 20(29)-Lupene-3,23-diol
Catalog No.:BCN5242
CAS No.:32451-85-7
- Isochlorogenic acid
Catalog No.:BCN5910
CAS No.:534-61-2
- NPS-2143 hydrochloride
Catalog No.:BCC1808
CAS No.:324523-20-8
- 1-Indanamine hydrochloride
Catalog No.:BCC8467
CAS No.:32457-23-1
- T 0156 hydrochloride
Catalog No.:BCC5803
CAS No.:324572-93-2
- Periplocymarin
Catalog No.:BCN8485
CAS No.:32476-67-8
Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation.[Pubmed:15131002]
Br J Pharmacol. 2004 May;142(1):161-71.
1. During mast cell degranulation, histamine is released in large quantities. Human eosinophils were found to express histamine H(4) but not H(3) receptors. The possible effects of histamine on eosinophils and the receptor mediating these effects were investigated in our studies. 2. Histamine (0.01-30 microm) induced a rapid and transient cell shape change in human eosinophils, but had no effects on neutrophils. The maximal shape change was at 0.3 microm histamine with EC(50) at 19 nm. After 60 min incubation with 1 microm histamine, eosinophils were desensitized and were refractory to shape change response upon histamine restimulation. Histamine (0.01-1 microm) also enhanced the eosinophil shape change induced by other chemokines. 3. Histamine-induced eosinophil shape change was mediated by the H(4) receptor. This effect was completely inhibited by H(4) receptor-specific antagonist JNJ 7777120 (IC(50) 0.3 microm) and H(3)/H(4) receptor antagonist thioperamide (IC(50) 1.4 microm), but not by selective H(1), H(2) or H(3) receptor antagonists. H(4) receptor agonists imetit (EC(50) 25 nm) and clobenpropit (EC(50) 72 nm) could mimic histamine effect in inducing eosinophil shape change. 4. Histamine (0.01-100 microm) induced upregulation of adhesion molecules CD11b/CD18 (Mac-1) and CD54 (ICAM-1) on eosinophils. This effect was mediated by the H(4) receptor and could be blocked by H(4) receptor antagonists JNJ 7777120 and thioperamide. 5. Histamine (0.01-10 microm) induced eosinophil chemotaxis with an EC(50) of 83 nm. This effect was mediated by the H(4) receptor and could be blocked by H(4) receptor antagonists JNJ 7777120 (IC(50) 86 nm) and thioperamide (IC(50) 519 nm). Histamine (0.5 microm) also enhanced the eosinophil shape change induced by other chemokines. 6. In conclusion, we have demonstrated a new mechanism of eosinophil recruitment driven by mast cells via the release of histamine. Using specific histamine receptor ligands, we have provided a definitive proof that the H(4) receptor mediates eosinophil chemotaxis, cell shape change and upregulation of adhesion molecules. The effect of H(4) receptor antagonists in blocking eosinophil infiltration could be valuable for the treatment of allergic diseases. The histamine-induced shape change and upregulation of adhesion molecules on eosinophils can serve as biomarkers for clinical studies of H(4) receptor antagonists.
Influence of different histamine receptor agonists and antagonists on apomorphine-induced licking behavior in rat.[Pubmed:10980276]
Eur J Pharmacol. 2000 Sep 15;404(1-2):169-74.
The effects of different histamine receptor agonists and antagonists on apomorphine-induced licking behavior in rats were investigated. Subcutaneous (s.c.) injection of various doses of apomorphine (0. 125-1.25 mg/kg) induced licking. The licking response was counted by direct observation and recorded for a 75-min period. Intracerebroventricular (i.c.v.) or intraperitoneal (i.p.) injection of the histamine H(1) or H(2) receptor agonist, HTMT (6-[2-(4-imidazolyl)ethylamino]-N-(4-trifluoromethylphenyl) heptanecarboxamide) (50 and 100 microg per rat), or dimaprit (10 and 15 mg/kg, i.p.), respectively, potentiated apomorphine-induced licking, while the histamine H(3) receptor agonist, imetit (5 and 10 mg/kg, i.p.), reduced the licking response induced by apomorphine. Pretreatment with various histamine receptor antagonists, dexchlorpheniramine (30 and 40 mg/kg, i.p.), diphenhydramine (20, 30 and 40 mg/kg, i.p.), famotidine (30 and 40 mg/kg, s.c.) and ranitidine (20, 30 and 40 mg/kg), reduced apomorphine-induced licking, while thioperamide (5 and 10 mg/kg, i.p.) potentiated the apomorphine effect. The effects of HTMT and dimaprit were blocked by dexchlorpheniramine (20 mg/kg, i.p.) and famotidine (20 mg/kg, s.c.), respectively. The inhibitory effect elicited by imetit on apomorphine-induced licking behavior was also abolished in animals treated with thioperamide (2.5 mg/kg, i.p.). The results suggest that histaminergic mechanisms may be involved in the modulation of apomorphine-induced licking behavior.
S-[2-(4-imidazolyl)ethyl]isothiourea, a highly specific and potent histamine H3 receptor agonist.[Pubmed:1383495]
J Pharmacol Exp Ther. 1992 Oct;263(1):304-10.
The effects of a new agonist of histamine (HA) H3 receptors, Imetit (S-[2-(4-(imidazolyl)ethyl]isothiourea) were investigated in vitro and in vivo and compared to those of (R)-alpha-methylhistamine [(R)-alpha-MeHA], a prototypic drug. Imetit inhibited the binding of [3H](R-alpha-MeHA to rat brain membranes with a Ki value of 0.1 +/- 0.01 nM. The release of endogenously synthesized [3H]HA induced by K(+)-depolarization from rat brain slices and synaptosomes was inhibited by Imetit with EC50 values of 1.0 +/- 0.3 and 2.8 +/- 0.7 nM, respectively. Imetit behaved as a full agonist and was about 4 times more potent than (R)-alpha-MeHA and 60 times more potent than HA. Thioperamide, a selective H3 receptor antagonist, elicited a parallel rightward shift of the concentration-response curve for Imetit with an apparent Ki value of 5.6 +/- 1.4 nM. Imetit potencies relative to HA were less than 0.1% and only 0.6% at HA H1 and H2 receptor reference systems, respectively. Imetit was found not to be a substrate or an inhibitor of HMT. After p.o. administration to mice or rats, Imetit decreased (by approximately 50%) the tele-MeHA level in the cerebral cortex with ED50 values of 1.0 +/- 0.3 and 1.6 +/- 0.3 mg/kg, respectively. This effect was still maximal after 6 hr. The in vivo potency and duration of action of Imetit were in the same range as those of (R)-alpha-MeHA. It is therefore concluded that Imetit represents a new potent and selective HA H3 receptor agonist.


