IsoastilbinCAS# 54081-48-0 |
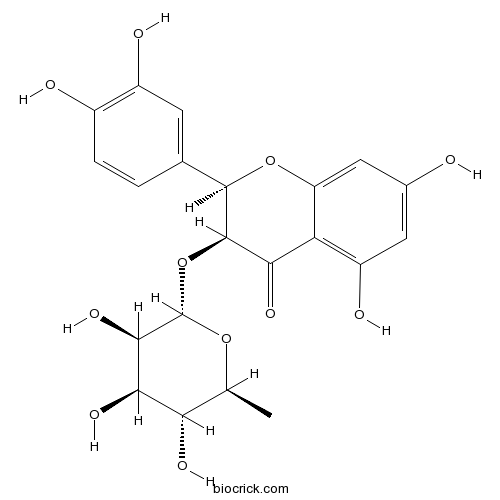
- Astilbin
Catalog No.:BCN5204
CAS No.:29838-67-3
- Neoisoastilbin
Catalog No.:BCN6532
CAS No.:54141-72-9
- Neoastilbin
Catalog No.:BCN8853
CAS No.:54081-47-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 54081-48-0 | SDF | Download SDF |
| PubChem ID | 9981176 | Appearance | Powder |
| Formula | C21H22O11 | M.Wt | 450.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3S)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-2,3-dihydrochromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OC2C(OC3=CC(=CC(=C3C2=O)O)O)C4=CC(=C(C=C4)O)O)O)O)O | ||
| Standard InChIKey | ZROGCCBNZBKLEL-OOHAXVOVSA-N | ||
| Standard InChI | InChI=1S/C21H22O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-26,28-29H,1H3/t7-,15-,17+,18+,19+,20+,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isoastilbin has anti-acne and tyrosinase inhibition properties.Isoastilbin, neoastilbin, astilbin and taxifolin have antimicrobial activities, they depress the growth of Streptococcus sobrinus (S. sobrinus) over a concentration range of 9.32-42.7 ug/ml and show GTase inhibitory activity with IC50 values in the range 27.4-57.3 ug/ml. |
| Targets | Antifection | Tyrosinase | GTase |
| In vitro | Antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of taxifolin and some flavanonol rhamnosides from kempas (Koompassia malaccensis) extracts[Reference: WebLink]J. Wood Sci., 2009, 55(4):308-13.Twenty plant materials collected from the islands of Java and Kalimantan in Indonesia were extracted with 50% aqueous ethanol (crude extract). Anti-acne and Tyrosinase Inhibition Properties of Taxifolin and Some Flavanonol Rhamnosides from Kempas ( Koompassia malaccensis )[Reference: WebLink]Wood Research Journal ,2010, 1(1):45-9.Taxifolin (1) and some flavanonol rhamnosides (neoastilbin (2), astilbin (3), and Isoastilbin (4)) have been isolated from kempas (Koompassia malaccensis). Our previous research about antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of these compounds have been reported. |
| Structure Identification | Yao Xue Xue Bao. 1996;31(10):761-3.Studies on the structure of isoastilbin.[Pubmed: 9863244]A new compound was isolated from Smilax glabra Roxb., named Isoastilbin. It was identified as 5, 7, 3', 5'-tetrahydroxyl-flavanonol-3-O-alpha-L-rhamnopyranoside by means of chemical and spectrometric analysis (UV, IR, 1H-NMR, 13C-NMR, 2DNMR and FAB-MS). |

Isoastilbin Dilution Calculator

Isoastilbin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2202 mL | 11.1012 mL | 22.2025 mL | 44.405 mL | 55.5062 mL |
| 5 mM | 0.444 mL | 2.2202 mL | 4.4405 mL | 8.881 mL | 11.1012 mL |
| 10 mM | 0.222 mL | 1.1101 mL | 2.2202 mL | 4.4405 mL | 5.5506 mL |
| 50 mM | 0.0444 mL | 0.222 mL | 0.444 mL | 0.8881 mL | 1.1101 mL |
| 100 mM | 0.0222 mL | 0.111 mL | 0.222 mL | 0.444 mL | 0.5551 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Palosuran
Catalog No.:BCC4311
CAS No.:540769-28-6
- Tofacitinib (CP-690550) Citrate
Catalog No.:BCC2189
CAS No.:540737-29-9
- Etonogestrel
Catalog No.:BCC5230
CAS No.:54048-10-1
- Albendazole Oxide
Catalog No.:BCC4757
CAS No.:54029-12-8
- Amifampridine
Catalog No.:BCC5185
CAS No.:54-96-6
- Pentylenetetrazole
Catalog No.:BCC7453
CAS No.:54-95-5
- Isoniazid
Catalog No.:BCC9003
CAS No.:54-85-3
- Cinanserin hydrochloride
Catalog No.:BCC6653
CAS No.:54-84-2
- Pilocarpine HCl
Catalog No.:BCC4702
CAS No.:54-71-7
- Idoxuridine
Catalog No.:BCC4666
CAS No.:54-42-2
- Metyrapone
Catalog No.:BCC7632
CAS No.:54-36-4
- Furosemide
Catalog No.:BCC3782
CAS No.:54-31-9
- 2-(1-Hydroxy-1-methylethyl)-4-methoxy-7H-furo[3,2-g][1]benzopyran-7-one
Catalog No.:BCN1422
CAS No.:54087-32-0
- L-Carnitine inner salt
Catalog No.:BCN1229
CAS No.:541-15-1
- Decamethonium Bromide
Catalog No.:BCC4568
CAS No.:541-22-0
- Isovaleramide
Catalog No.:BCC4668
CAS No.:541-46-8
- Muscone
Catalog No.:BCN6275
CAS No.:541-91-3
- 15-Hydroxydehydroabietic acid
Catalog No.:BCN5720
CAS No.:54113-95-0
- 9-Benzylcarbazole-3-carboxaldehyde
Catalog No.:BCC8800
CAS No.:54117-37-2
- Apoptosis Inhibitor
Catalog No.:BCC1143
CAS No.:54135-60-3
- Neoisoastilbin
Catalog No.:BCN6532
CAS No.:54141-72-9
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Vicriviroc Malate
Catalog No.:BCC1230
CAS No.:541503-81-5
- Apilimod
Catalog No.:BCC5286
CAS No.:541550-19-0
[Studies on the structure of isoastilbin].[Pubmed:9863244]
Yao Xue Xue Bao. 1996;31(10):761-3.
A new compound was isolated from Smilax glabra Roxb., named Isoastilbin. It was identified as 5, 7, 3', 5'-tetrahydroxyl-flavanonol-3-O-alpha-L-rhamnopyranoside by means of chemical and spectrometric analysis (UV, IR, 1H-NMR, 13C-NMR, 2DNMR and FAB-MS).


