Justicidin BCAS# 17951-19-8 |
Quality Control & MSDS
Number of papers citing our products

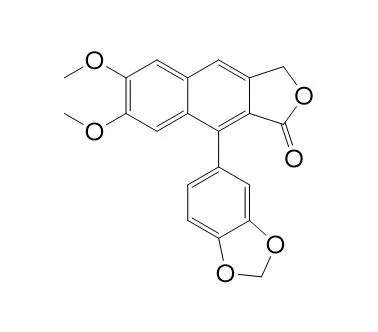
Chemical structure

| Cas No. | 17951-19-8 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C21H16O6 | M.Wt | 364.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Justicidin B Dilution Calculator

Justicidin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7442 mL | 13.7212 mL | 27.4424 mL | 54.8847 mL | 68.6059 mL |
| 5 mM | 0.5488 mL | 2.7442 mL | 5.4885 mL | 10.9769 mL | 13.7212 mL |
| 10 mM | 0.2744 mL | 1.3721 mL | 2.7442 mL | 5.4885 mL | 6.8606 mL |
| 50 mM | 0.0549 mL | 0.2744 mL | 0.5488 mL | 1.0977 mL | 1.3721 mL |
| 100 mM | 0.0274 mL | 0.1372 mL | 0.2744 mL | 0.5488 mL | 0.6861 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- threo-7,9,9'-Trihydroxy-3,3'-dimethoxy-8-O-4'-neolignan 4-O-beta-D-glucopyranoside
Catalog No.:BCX0151
CAS No.:1009838-88-3
- Dehydropipernonaline
Catalog No.:BCX0150
CAS No.:107584-38-3
- Pyrolaside B analogue
Catalog No.:BCX0149
CAS No.:1053610-99-3
- 2,4,12-Octadecatrienoic acid isobutylamide
Catalog No.:BCX0148
CAS No.:151391-69-4
- Jatairidoid A
Catalog No.:BCX0147
CAS No.:1393577-29-1
- 3-Hydroxy-4',5,7-trimethoxyflavan
Catalog No.:BCX0146
CAS No.:3143-21-3
- 7-(Hydroxymethyl)-2-methyl-1,4-naphthalenedione
Catalog No.:BCX0145
CAS No.:145626-36-4
- Methyl piperate
Catalog No.:BCX0144
CAS No.:6190-46-1
- Periclymenosidic acid
Catalog No.:BCX0143
CAS No.:96681-56-0
- 4,7,9,9'-Tetrahydroxy-3,3'-dimethoxy-8,4'-oxyneolignan 7-O-beta-D-glucopyranoside
Catalog No.:BCX0142
CAS No.:182056-97-9
- Pipernonaline
Catalog No.:BCX0141
CAS No.:88660-10-0
- Pyrolaside B
Catalog No.:BCX0140
CAS No.:868632-32-0
- Chinensinaphthol methyl ether
Catalog No.:BCX0153
CAS No.:53965-11-0
- Justicidin D
Catalog No.:BCX0154
CAS No.:27041-98-1
- Isorinic acid
Catalog No.:BCX0155
CAS No.:145757-60-4
- Melliferone
Catalog No.:BCX0156
CAS No.:377724-68-0
- Huzhangoside C
Catalog No.:BCX0157
CAS No.:96315-52-5
- Procumbenoside E
Catalog No.:BCX0158
CAS No.:220182-12-7
- Kaempferol 3-(2''-galloylglucoside)
Catalog No.:BCX0159
CAS No.:76343-90-3
- Lapathoside A
Catalog No.:BCX0160
CAS No.:373646-49-2
- Justicidin C
Catalog No.:BCX0161
CAS No.:17803-12-2
- Massonianoside A
Catalog No.:BCX0162
CAS No.:623945-11-9
- Justicidinoside C
Catalog No.:BCX0163
CAS No.:177912-23-1
- Retrofractamide C
Catalog No.:BCX0164
CAS No.:96386-33-3
In Silico Exploration of Potential Phytoconstituents from the Bark Extract of Boswellia serrata for Hemorrhoidal Disease: Molecular Docking and Molecular Dynamics Analysis.[Pubmed:38078787]
Chem Biodivers. 2023 Dec 11:e202301416.
Boswellia serrata Roxb. Ex Colebr is a popular medicinal plant used traditionally in herbal medicinal preparations to treat a variety of diseases. The purpose of the present investigation was to investigate the anti-hemorrhoidal property of the bark extract of B. serrata (BS). For this, the sequential Soxhlet extraction method was carried out by using different solvents such as hexane, chloroform, and methanol. After the extraction, the obtained dry extracts were tested for quantitative determinations such as total alkaloid content (TAC), total flavonoid content (TFC), total phenol content (TPC), and total tannin content (TTC) for all the extracts. Moreover, in vitro antioxidant activity was measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity and scavenging activity against 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS). Methanolic bark extract showed the highest TPC (67.10+/-1.83), TFC (372.73+/-4.45), TAC (9.732+/-1.06), and TTC (48.932+/-1.82), as well as the antioxidant assays DPPH (IC50=9.88 mug/ml) and ABTS (IC50=15.09 mug/ml). In this study, both LC-MS and GC-MS were performed to identify the chemical composition of all the extracts. Consequently, 19 compounds were identified by GC-MS and 27 compounds were identified by LC-MS analysis. The identified phytoconstituent(s) that could potentially interact with the target protein cyclooxygenase-2 (COX-2) (PDB: 4RRW) using molecular dynamics simulation and in silico docking were studied. Three compounds that have passed in drug-likeness and ADME-Tox properties are having more docking score than the standard. In this study, camptothecin, Justicidin B, and taxiphyllin are identified as potential lead compounds with anti-hemorrhoidal properties and may be helpful in the process of drug development and discovery of novel drugs. Hence, these results demonstrate that BS is a good source of pharmacologically active components with potential applications against hemorrhoidal disease.
Exploring the antiviral potential of justicidin B and four glycosylated lignans from Phyllanthus brasiliensis against Zika virus: A promising pharmacological approach.[Pubmed:37952409]
Phytomedicine. 2023 Nov 7;123:155197.
BACKGROUND: Zika virus (ZIKV) is an emerging arbovirus that in recent years has been associated with cases of severe neurological disorders, such as microcephaly in newborns and Guillain-Barre syndrome in adults. As there is no vaccine or treatment, the search for new therapeutic targets is of great relevance. In this sense, plants are extremely rich sources for the discovery of new bioactive compounds and the species Phyllanthus brasiliensis (native to the Amazon region) remains unexplored. PURPOSE: To investigate the potential antiviral activity of compounds isolated from P. brasiliensis leaves against ZIKV infection. METHODS: In vitro antiviral assays were performed with Justicidin B (a lignan) and four glycosylated lignans (tuberculatin, phyllanthostatin A, 5-O-beta-d-glucopyranosylJusticidin B, and cleistanthin B) against ZIKV in Vero cells. MTT colorimetric assay was used to assess cell viability and plaque forming unit assay to quantify viral load. In addition, for Justicidin B, tests were performed to investigate the mechanism of action (virucidal, adsorption, internalization, post-infection). RESULTS: The isolated compounds showed potent anti-ZIKV activities and high selectivity indexes. Moreover, Justicidin B, tuberculatin, and phyllanthostatin A completely reduced the viral load in at least one of the concentrations evaluated. Among them, Justicidin B stood out as the main active, and further investigation revealed that Justicidin B exerts its antiviral effect during post-infection stages, resulting in a remarkable 99.9 % reduction in viral load when treatment was initiated 24 h after infection. CONCLUSION: Our findings suggest that Justicidin B inhibits endosomal internalization and acidification, effectively interrupting the viral multiplication cycle. Therefore, the findings shed light on the promising potential of isolated compounds isolated from P. brasiliensis, especially Justicidin B, which could contribute to the drug development and treatments for Zika virus infections.
Molecular Docking and Molecular Dynamics Studies Reveal the Anticancer Potential of Medicinal-Plant-Derived Lignans as MDM2-P53 Interaction Inhibitors.[Pubmed:37764441]
Molecules. 2023 Sep 16;28(18):6665.
The interaction between the tumor suppressor protein p53 and its negative regulator, the MDM2 oncogenic protein, has gained significant attention in cancer drug discovery. In this study, 120 lignans reported from Ferula sinkiangensis and Justicia procumbens were assessed for docking simulations on the active pocket of the MDM2 crystal structure bound to Nutlin-3a. The docking analysis identified nine compounds with higher docking scores than the co-crystallized reference. Subsequent AMDET profiling revealed satisfactory pharmacokinetic and safety parameters for these natural products. Three compounds, namely, justin A, 6-hydroxy justicidin A, and 6'-hydroxy Justicidin B, were selected for further investigation due to their strong binding affinities of -7.526 kcal/mol, -7.438 kcal/mol, and -7.240 kcal/mol, respectively, which surpassed the binding affinity of the reference inhibitor Nutlin-3a (-6.830 kcal/mol). To assess the stability and reliability of the binding of the candidate hits, a molecular dynamics simulation was performed over a duration of 100 ns. Remarkably, the thorough analysis demonstrated that all the hits exhibited stable molecular dynamics profiles. Based on their effective binding to MDM2, favorable pharmacokinetic properties, and molecular dynamics behavior, these compounds represent a promising starting point for further refinement. Nevertheless, it is essential to synthesize the suggested compounds and evaluate their activity through in vitro and in vivo experiments.
Secondary Metabolites Profiling, Antimicrobial and Cytotoxic Properties of Commiphora gileadensis L. Leaves, Seeds, Callus, and Cell Suspension Extracts.[Pubmed:37110196]
Metabolites. 2023 Apr 10;13(4):537.
Commiphora gileadensis L. is an important endangered medicinal plant that belongs to the family Burseraceae. In this study, C. gileadensis callus culture was established successfully using mature leaves as explants cultured on Murashige and Skoog (MS) media supplemented with 24.50 muM of indole butyric acid (IBA) and 2.22 muM 6-Benzylaminopurine (BAP) (callus induction media). The obtained callus was maintained on MS medium supplemented with 16.11 muM naphthalene acetic acid (NAA) in combination with 6.66 muM BAP, which resulted in a substantial increase in callus fresh and dry weights. The cell suspension culture was established successfully using liquid callus induction media supplemented with 3.0 mg.L(-1) proline. Thereafter, the chemical constituents of different C. gileadensis methanolic extracts (callus, cell suspension, leaves, and seeds) were profiled, and their cytotoxic and antimicrobial properties were investigated. The LC-MS GNPS analyses were applied for chemical profiling of the methanolic plant extracts, and several natural products were identified, including flavonols, flavanones, and flavonoids glycosides, with two unusual families that included puromycin, 10-hydroxycamptothecin, and Justicidin B. The methanolic extracts have shown selective antimicrobial and cytotoxic properties against different microbes and cancer cell lines. For instance, leaf extract showed the highest zone of inhibition for Staphylococcus aureus, while cell suspension culture was effective against Staphylococcus epidermidis and Staphylococcus aureus. All extracts showed selective activity against A549 cell lines for the cytotoxicity assay, while the leaf extract had a broad cytotoxic effect against all tested cell lines. This study revealed that C. gileadensis callus and cell suspension cultures can be employed to increase the in vitro formation of biologically active compounds that may have cytotoxicity and antibacterial action against different cancer cell lines and bacterial species. Further studies are required to isolate and identify such constituents that corroborate the observed activities.
Arylnaphthalene Lignans with Anti-SARS-CoV-2 and Antiproliferative Activities from the Underground Organs of Linum austriacum and Linum perenne.[Pubmed:36857518]
J Nat Prod. 2023 Apr 28;86(4):672-682.
Diphyllin (1) and Justicidin B (2) are arylnaphthalene lignans with antiviral and antiproliferative effects. Compound 1 is also known as an effective inhibitor of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). To evaluate the in vitro antiviral and cytotoxic potency of both lignans in SARS-CoV-2 -infected cells and various cancer cell lines, respectively, 1 and 2 were isolated from the underground organs of Linum austriacum and Linum perenne. Two previously undescribed arylnaphthalene lignans, denominated linadiacin A and B (3 and 4), were also isolated and identified. In acidic media, 3 was converted by a two-step reaction into 2 via the intermediate 4. Optimum acid treatment conditions were determined to isolate lignans by one-step preparative high-performance liquid chromatography (HPLC). The results of the conversion, HPLC-tandem mass spectrometry, nuclear magnetic resonance spectroscopy, and molecular modeling studies allowed complete structure analysis. Compounds 1 and 2 were the most effective against SARS-CoV-2 with a 3-log reduction in the viral copy number at a 12.5 muM concentration. Ten human cancer cell lines showed sensitivity to at least one of the isolated lignans.
Identifying bioactive phytoconstituents as C-terminal Src kinase inhibitors: a virtual screening and molecular simulation approach.[Pubmed:36752377]
J Biomol Struct Dyn. 2023;41(22):13415-13424.
Tyrosine-protein kinase CSK otherwise known as C-terminal Src kinase (CSK), is involved in multiple pathways and processes, including regulating cell growth, differentiation, migration, and immune responses. Altered expression of CSK has been associated with various complexities, including cancer, CD45 deficiency, Osteopetrosis and lupus erythematosus. Important auxiliary roles of CSK in cancer progression make it a crucial target in developing novel anticancer therapy. Thus, CSK inhibitors are of concern as potent immuno-oncology agents. In this perspective, phytochemicals can be a significant source for unraveling novel CSK inhibitors. In this study, we carried out a systematic structure-based virtual screening of bioactive phytoconstituents against CSK to identify its potential inhibitors. After a multi-step screening process, two hits (Shinpterocarpin and Justicidin B) were selected based on their druglike properties and binding affinity towards CSK. The selected hits were further analyzed for their stability and interaction via all-atom molecular dynamics (MD) simulations. The selected hits indicated their potential as selective binding partners of CSK, which can further be used for therapeutic development against CSK-associated malignancies.Communicated by Ramaswamy H. Sarma.
Total synthesis of justicidin B, justicidin E, and taiwanin C: A general and flexible approach toward the synthesis of natural arylnaphthalene lactone lignans.[Pubmed:36618865]
Front Chem. 2022 Dec 22;10:1103554.
Lignans are widely present in traditional medicinal plants. Many natural arylnaphthalene lactone lignans (NALLs) isolated from the genera Justicia, Haplophyllum, and Phyllanthus possess interesting biological activities. Herein, we report a general strategy for the total synthesis of this kind of lignans. Features of this new approach are an aryl-alkyl Suzuki cross-coupling to introduce the dioxinone unit, a cation-induced cyclization to construct the aryl dihydronaphthalene, and base-mediated oxidative aromatization to furnish the arylnaphthalene core. By incorporating these key transformations, the total syntheses of justicidins B and E and taiwanin C covered type I and type II NALLs were accomplished.
Linum lewisii Adventitious and Hairy-Roots Cultures as Lignan Plant Factories.[Pubmed:36009248]
Antioxidants (Basel). 2022 Aug 5;11(8):1526.
Plants synthesize specific secondary metabolites for survival, reproduction, environmental resilience, and defense. Among them, lignans are a class of polyphenols with several bioactive properties: chemopreventive, anti-inflammatory, antiviral, and antioxidant. These compounds are often extracted from field-grown plants with very low yields. To overcome these constraints, in vitro tissue cultures provide a tool to optimize large-scale production. Moreover, the use of elicitation to increase secondary metabolite production is gaining importance. The aim of this work was to develop adventitious (ARL) and hairy roots (HRL) from Linum lewisi, a species able to synthesize arylnaphthalene lignans such as Justicidin B. The ARL and HRL were obtained for the first time and characterized for their phenol content, antioxidant activity, and the production of Justicidin B after treatments with several elicitors and precursor feeding. Through NMR spectroscopy, other four lignans were highlighted and identified in the roots extracts. A pilot-scale bioreactor was adopted to assess the suitability of the developed root cultures for future large-scale production. The ARL and HRL cultures showed a Justicidin B production higher than other Linum species cultures described up to now (75.8 mg/L and 82.2 g/L), and the production more than doubled after elicitation with MeJA.
Effective components and mechanism analysis of anti-platelet aggregation effect of Justicia procumbens L.[Pubmed:35589019]
J Ethnopharmacol. 2022 Aug 10;294:115392.
ETHNOPHARMACOLOGICAL RELEVANCE: Justicia procumbens L. is a traditional Chinese medicine, first recorded in "Shen Nong's Herbal Classic", for the treatment of lumbar pain and fever. As a widely distributed herb, it has also been documented in India, Nepal, and Malaysia. In "Tang Materia Medica", a famous medicinal book of Tang Dynasty in ancient China, it was first used to treat diseases associated with blood stasis. Blood stasis syndrome is closely related to thrombus formation and platelet aggregation. Although some compounds isolated from this plant have anti-platelet aggregation effects, the main chemical components and mechanism of J. procumbens in terms of these effects are little known. AIMS OF THE STUDY: Through in vivo and in vitro experiments, this studsy revealed the characteristic components and action mechanism of anti-platelet aggregation by J. procumbens from an overall perspective. MATERIALS AND METHODS: The effective crude extracts of the whole plant were screened via an in vitro anti-platelet aggregation test. After incubating these extracts with apheresis platelets, high affinity compounds were detected by HPLC-MS and regulatory genes were detected using gene chips. The effective components and potential target proteins were analyzed using computational docking technology. Furthermore, the compound with the strongest predicted activity was evaluated in vivo via an anti-thrombotic test. RESULTS: Integrin a(Ⅱb)beta(3), PKCalpha, PI3Kgamma, and mitogen-activated protein kinase 14 were found to be potential targets. Justicidin B, tuberculatin, chinensinaphthol methyl ether, and neojusticin B were effective compounds that inhibited human platelet aggregation by suppressing Gq-PLC-PKC and Gi-PI3K-MAPK signaling pathways. Among the compounds that bind to platelets, Justicidin B showed the strongest virtual binding force. The test of carotid artery thrombosis induced by ferric chloride in SD rats confirmed that Justicidin B inhibited thrombus formation. CONCLUSION: Experimental investigation showed that arylnaphthalene lignan aglycones with one methylenedioxy group and two methoxy groups are effective components for anti-platelet aggregation by J. procumbens. These compounds inhibit Gq-PLC-PKC and Gi-PI3K-MAPK signaling pathways by suppressing the expression of genes such as ITGB3, PRKCA, PIK3CG, and MAPK14. These results reflected the characteristics of multi-component and multi-target synergistic treatment of Chinese medicine.
Optimized Ultrasound-Assisted Extraction of Lignans from Linum Species with Green Solvents.[Pubmed:35566080]
Molecules. 2022 Apr 23;27(9):2732.
Lignans are plant phenols derived from phenylpropanoids. They play a significant role in plant defense and have features that make them appealing for pharmaceutical applications. Lignans can be obtained by plant in vitro cultures; their production by adventitious and hairy roots of Linum species seems to be a promising alternative to chemical synthesis. In the context of large-scale production, it is necessary to optimize their extraction from plants tissue by choosing the more suitable solvent and extraction procedure, paying attention to the use of green media and methods. With the aim to select the best conditions for the extraction of two interesting lignans (Justicidin B and 6-methoxypodophyllotoxin) from Linum tissues, different green solvents and the method of ultrasound-assisted extraction were tested. The results showed that ethyl methyl ketone and dimethyl carbonate were the best media to extract Justicidin B and 6-methoxypodophyllotoxin, respectively, in terms of purity and recovery. Moreover, we showed that ultrasound-assisted extraction presents different advantages compared to conventional methods. Finally, the optimal experimental conditions to extract Justicidin B from L. austriacum hairy roots using methyl ethyl ketone were also determined by the response surface method. The models obtained are reliable and accurate to estimate the purity and recovery of Justicidin B.
The efficient synthesis and biological evaluation of justicidin B.[Pubmed:34227447]
Nat Prod Res. 2023 Jan;37(1):56-62.
A facile new synthetic method for the preparation of a Type-A 1-arylnaphthalene lactone skeleton was developed and used to synthesise Justicidin B and several derivatives. Key synthesis steps included Hauser-Kraus annulation of a phthalide intermediate and Suzuki-Miyaura cross coupling between a triflated naphthalene lactone intermediate and various potassium organotrifluoroborates. With two exceptions, the derivatives showed significant inhibitory effect on lipopolysaccharide (LPS)-induced nitric oxide (NO) production in mouse macrophages. Moreover, several compounds, including Justicidin B, had marked cytotoxicity towards six human tumour cell lines.
New Insight into Justicidin B Pathway and Production in Linum austriacum.[Pubmed:33801525]
Int J Mol Sci. 2021 Mar 2;22(5):2507.
Lignans are the main secondary metabolites synthetized by Linum species as plant defense compounds but they are also valuable for human health, in particular, for novel therapeutics. In this work, Linum austriacum in vitro cultures, cells (Cc), adventitious roots (ARc) and hairy roots (HRc) were developed for the production of Justicidin B through elicitation with methyl jasmonate (MeJA) and coronatine (COR). The performances of the cultures were evaluated for their stability, total phenols content and antioxidant ability. NMR was used to identify Justicidin B and isoJusticidin B and HPLC to quantify the production, highlighting ARc and HRc as the highest productive tissues. MeJA and COR treatments induced the synthesis of Justicidin B more than three times and the synthesis of other compounds. RNA-sequencing and a de novo assembly of L. austriacum ARc transcriptome was generated to identify the genes activated by MeJA. Furthermore, for the first time, the intracellular localization of Justicidin B in ARc was investigated through microscopic analysis. Then, HRc was chosen for small-scale production in a bioreactor. Altogether, our results improve knowledge on Justicidin B pathway and cellular localization in L. austriacum for future scale-up processes.
Design, synthesis, and evaluation of cytotoxic activities of arylnaphthalene lignans and aza-analogs.[Pubmed:33586249]
Arch Pharm (Weinheim). 2021 Jun;354(6):e2000479.
A concise and versatile synthetic strategy for the total synthesis of arylnaphthalene lignans and aza-analogs was developed. The main objective was to develop synthetic tactics for the creation of the lactone and lactam unit that would give access to an array of synthetic, natural, and/or bioactive compounds through rather simple chemical manipulation. The flexibility and potentiality of these new processes were further illustrated by the total synthesis of retroJusticidin B (13b), justicidin C (14b), and methoxy-vitedoamine A (22a). In this study, a series of novel aryl-naphthalene lignans and aza-analogs were synthesized, and the cytotoxic activities of all compounds on cancer cell growth were evaluated. The target compounds were structurally characterized by (1) H NMR (nuclear magnetic resonance), (13) C NMR, infrared, high-resolution mass spectrometry, and X-ray crystallography. The IC(50) values of these compounds on five tumor cell lines (A549, HS683, MCF-7, SK-MEL-28, and B16-F1) were obtained by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay. Five of the compounds exhibited excellent activity compared to 5-fluorouracil and etoposide against the five cell lines tested, with IC(50) values ranging from 1 to 10 muM.
Comparative Metabolite Profiling of Wild and Cultivated Justicia procumbens L. Based on (1)H-NMR Spectroscopy and HPLC-DAD Analysis.[Pubmed:32646001]
Plants (Basel). 2020 Jul 7;9(7):860.
Justicia procumbens L. is known across Korea, India, China, and Taiwan as a remedy against fever, cough, sore throat, and cirrhosis of ascites. J. procumbens provides the raw material for a candidate anti-asthma drug (DW2008S) currently completing phase I clinical trials sponsored by Dong Wha Pharmaceutical Company. HPLC-DAD was used to quantify phytochemical constituents of J. procumbens, and HPLC and (1)H-NMR results were assessed by multivariate analysis. This is the first time a comparative study using HPLC-DAD and NMR fingerprints has been applied to identify chemical differences between wild and cultivated J. procumbens. The amount of Justicidin B as the marker compound was higher in cultivated samples (0.80 +/- 0.25 mg/g) than in wild ones (0.63 +/- 0.30 mg/g). Orthogonal partial least squares discriminant analysis (OPLS-DA) from HPLC and NMR data revealed that there were clear differences between wild and cultivated types and identified five secondary metabolites, which could help distinguish between wild and cultivated plants. Among these five lignans, diphyllin showed the most potent discrimination between two types and was significantly detected higher in cultivated ones than in wild ones. A combination of (1)H-NMR and HPLC-DAD analysis is effective for J. procumbens standardization and metabolomics studies.


