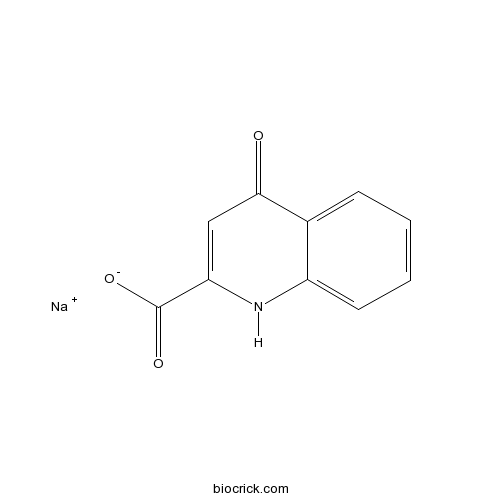
Kynurenic acid sodium saltCAS# 2439-02-3 |
- 3-Deazaneplanocin,DZNep
Catalog No.:BCC1129
CAS No.:102052-95-9
- 3-Deazaneplanocin A (DZNep) hydrochloride
Catalog No.:BCC3604
CAS No.:120964-45-6
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- PluriSIn #1 (NSC 14613)
Catalog No.:BCC2305
CAS No.:91396-88-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 2439-02-3 | SDF | Download SDF |
| PubChem ID | 52974250 | Appearance | Powder |
| Formula | C10H6NNaO3 | M.Wt | 211.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Kynurenic Acid Sodium Salt; Sodium Kynurenate | ||
| Solubility | DMSO : 50 mg/mL (235.67 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | sodium;4-oxo-1H-quinoline-2-carboxylate | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C=C(N2)C(=O)[O-].[Na+] | ||
| Standard InChIKey | RCAZGXKUQDXSSK-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C10H7NO3.Na/c12-9-5-8(10(13)14)11-7-4-2-1-3-6(7)9;/h1-5H,(H,11,12)(H,13,14);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sodium salt of kynurenic acid, a broad spectrum EAA antagonist. |

Kynurenic acid sodium salt Dilution Calculator

Kynurenic acid sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.736 mL | 23.6798 mL | 47.3597 mL | 94.7194 mL | 118.3992 mL |
| 5 mM | 0.9472 mL | 4.736 mL | 9.4719 mL | 18.9439 mL | 23.6798 mL |
| 10 mM | 0.4736 mL | 2.368 mL | 4.736 mL | 9.4719 mL | 11.8399 mL |
| 50 mM | 0.0947 mL | 0.4736 mL | 0.9472 mL | 1.8944 mL | 2.368 mL |
| 100 mM | 0.0474 mL | 0.2368 mL | 0.4736 mL | 0.9472 mL | 1.184 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5-Iodotubercidin
Catalog No.:BCC1312
CAS No.:24386-93-4
- Glycoside L-F2
Catalog No.:BCN2158
CAS No.:243857-99-0
- pep4c
Catalog No.:BCC5783
CAS No.:243843-43-8
- pep2m
Catalog No.:BCC5782
CAS No.:243843-42-7
- L-(-)-Fucose
Catalog No.:BCN8326
CAS No.:2438-80-4
- Bufexamac
Catalog No.:BCC4427
CAS No.:2438-72-4
- (-)-alpha-Pinene
Catalog No.:BCC8295
CAS No.:2437-95-8
- 3,5-Cycloergosta-6,8(14),22-triene
Catalog No.:BCN5100
CAS No.:24352-51-0
- S-(5'-Adenosyl)-L-methionine chloride
Catalog No.:BCN2229
CAS No.:24346-00-7
- Apamin
Catalog No.:BCC7141
CAS No.:24345-16-2
- 6-Amino-1-methyluracil
Catalog No.:BCC8757
CAS No.:2434-53-9
- Nagilactone C
Catalog No.:BCN4040
CAS No.:24338-53-2
- Ethyl 4-methoxycinnamate
Catalog No.:BCN5028
CAS No.:24393-56-4
- Cnicin
Catalog No.:BCN8546
CAS No.:24394-09-0
- TCS 401
Catalog No.:BCC2469
CAS No.:243967-42-2
- TAK-242 S enantiomer
Catalog No.:BCC1978
CAS No.:243984-10-3
- TAK-242
Catalog No.:BCC1977
CAS No.:243984-11-4
- beta-D-glucose
Catalog No.:BCN8171
CAS No.:492-61-5
- (+)-Epipinoresinol
Catalog No.:BCN3255
CAS No.:24404-50-0
- Beta-Rotunol
Catalog No.:BCN6628
CAS No.:24405-57-0
- L-798,106
Catalog No.:BCC7654
CAS No.:244101-02-8
- Taxumairol R
Catalog No.:BCN6939
CAS No.:244167-04-2
- L-748,337
Catalog No.:BCC7475
CAS No.:244192-94-7
- Pulchinenoside E2
Catalog No.:BCN8186
CAS No.:244202-36-6
Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35.[Pubmed:16754668]
J Biol Chem. 2006 Aug 4;281(31):22021-8.
Local catabolism of the essential amino acid tryptophan is considered an important mechanism in regulating immunological and neurological responses. The kynurenine pathway is the main route for the non-protein metabolism of tryptophan. The intermediates of the kynurenine pathway are present at micromolar concentrations in blood and are regulated by inflammatory stimuli. Here we show that GPR35, a previously orphan G protein-coupled receptor, functions as a receptor for the kynurenine pathway intermediate kynurenic acid. Kynurenic acid elicits calcium mobilization and inositol phosphate production in a GPR35-dependent manner in the presence of G(qi/o) chimeric G proteins. Kynurenic acid stimulates [35S]guanosine 5'-O-(3-thiotriphosphate) binding in GPR35-expressing cells, an effect abolished by pertussis toxin treatment. Kynurenic acid also induces the internalization of GPR35. Expression analysis indicates that GPR35 is predominantly detected in immune cells and the gastrointestinal tract. Furthermore, we show that kynurenic acid inhibits lipopolysaccharide-induced tumor necrosis factor-alpha secretion in peripheral blood mononuclear cells. Our results suggest unexpected signaling functions for kynurenic acid through GPR35 activation.
The "kynurenate test", a biochemical assay for putative cognition enhancers.[Pubmed:9336311]
J Pharmacol Exp Ther. 1997 Oct;283(1):82-90.
Some putative cognition enhancers (oxiracetam, aniracetam and D-cycloserine) were previously shown to prevent the kynurenic acid antagonism of the N-methyl-D-aspartate (NMDA)-evoked norepinephrine (NE) release in rat hippocampal slices. This functional in vitro assay was further characterized in the present work. D-Serine, a glutamate coagonist at the NMDA receptor glycine site, concentration-dependently (EC50 approximately 0.1 microM) prevented the kynurenate (100 microM) block of the NMDA (100 microM)-evoked [3H]NE release. L-Serine was ineffective up to 10 microM. The gamma-aminobutyric acidB (GABA[B]) receptor antagonist CGP 36742, reported to improve cognitive performance, potently prevented the kynurenate antagonism. The activity of CGP 36742 (1 microM) appeared to be unaffected by 10 microM (-)-baclofen, a GABA(B) receptor agonist; furthermore, CGP 52432, a GABA(B) antagonist more potent than CGP 36742, but reportedly devoid of nootropic properties, was inactive in the "kynurenate test." The novel putative cognition enhancer CR2249, but not its enantiomer CR2361, also potently prevented the kynurenate antagonism. In contrast, linopirdine, nicotine and tacrine were inactive. In rat hippocampal synaptosomes glycine and D-cycloserine enhanced the NMDA-evoked [3H]NE release, whereas oxiracetam and CR2249 did not. These four compounds were all similarly effective in preventing kynurenate antagonism, both in slices and in synaptosomes. The NMDA potentiation caused by glycine (0.1-100 microM) was not affected by 100 microM oxiracetam, which suggested that drugs active in the "kynurenate test" may bind to sites different from the glycine site of the NMDA receptor. To conclude, the "kynurenate test" is an in vitro assay useful in the identification and characterization of putative cognition enhancers acting via NMDA receptors.


