Locustatachykinin IInsect tachykinin-related peptide (TRP) CAS# 126985-97-5 |
- Apicidin
Catalog No.:BCC3599
CAS No.:183506-66-3
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 126985-97-5 | SDF | Download SDF |
| PubChem ID | 195503 | Appearance | Powder |
| Formula | C43H63N13O11 | M.Wt | 938.05 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Lom-TK I | ||
| Solubility | Soluble to 1 mg/ml in water | ||
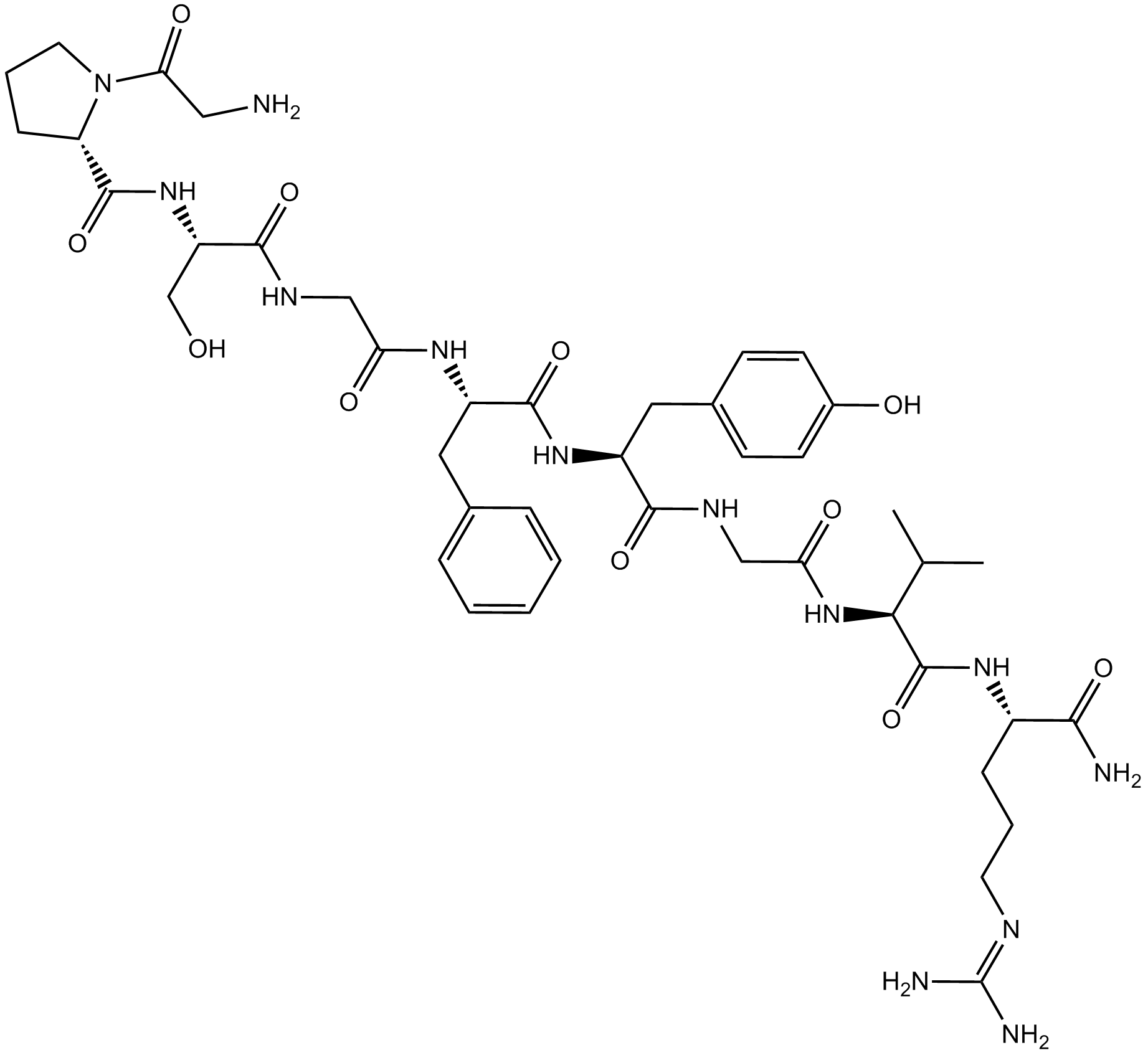
| Sequence | GPSGFYGVR (Modifications: Arg-9 = C-terminal amide) | ||
| Chemical Name | (2S)-1-(2-aminoacetyl)-N-[(2S)-1-[[2-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[[(2S)-1-amino-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-2-oxoethyl]amino]-3-hydroxy-1-oxopropan-2-yl]pyrrolidine-2-carboxamide | ||
| SMILES | CC(C)C(C(=O)NC(CCCN=C(N)N)C(=O)N)NC(=O)CNC(=O)C(CC1=CC=C(C=C1)O)NC(=O)C(CC2=CC=CC=C2)NC(=O)CNC(=O)C(CO)NC(=O)C3CCCN3C(=O)CN | ||
| Standard InChIKey | QVHUTTNZGVKOKQ-LINCNNNZSA-N | ||
| Standard InChI | InChI=1S/C43H63N13O11/c1-24(2)36(42(67)52-28(37(45)62)10-6-16-48-43(46)47)55-34(60)22-49-38(63)29(19-26-12-14-27(58)15-13-26)53-40(65)30(18-25-8-4-3-5-9-25)51-33(59)21-50-39(64)31(23-57)54-41(66)32-11-7-17-56(32)35(61)20-44/h3-5,8-9,12-15,24,28-32,36,57-58H,6-7,10-11,16-23,44H2,1-2H3,(H2,45,62)(H,49,63)(H,50,64)(H,51,59)(H,52,67)(H,53,65)(H,54,66)(H,55,60)(H4,46,47,48)/t28-,29-,30-,31-,32-,36-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Insect tachykinin-related peptide (TRP) isolated from Locusta migratoria. Exhibits sequence homology with vertebrate tachykinins. |

Locustatachykinin I Dilution Calculator

Locustatachykinin I Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-(3,4-Dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane-3,5-diyl diacetate
Catalog No.:BCN6572
CAS No.:1269839-26-0
- 5-Hydroxy-1,7-bis(4-hydroxyphenyl)heptan-3-yl acetate
Catalog No.:BCN6586
CAS No.:1269839-24-8
- CDK inhibitor II
Catalog No.:BCC1464
CAS No.:1269815-17-9
- Tetrahydro tanshinone I
Catalog No.:BCN2602
CAS No.:126979-84-8
- Methylenedihydrotanshinquinone
Catalog No.:BCN3221
CAS No.:126979-81-5
- Sar-[D-Phe8]-des-Arg9-Bradykinin
Catalog No.:BCC5996
CAS No.:126959-88-4
- LGX818
Catalog No.:BCC4184
CAS No.:1269440-17-6
- MK 1903
Catalog No.:BCC6242
CAS No.:1268882-43-4
- 3-Methoxy-5-heneicosylphenol
Catalog No.:BCN6147
CAS No.:126882-76-6
- Ssioriside
Catalog No.:BCN6146
CAS No.:126882-53-9
- MC 1046
Catalog No.:BCC1733
CAS No.:126860-83-1
- (-)-JQ1
Catalog No.:BCC3603
CAS No.:1268524-71-5
- Hydroxyurea
Catalog No.:BCC4912
CAS No.:127-07-1
- Sodium acetate
Catalog No.:BCC7587
CAS No.:127-09-3
- Taraxerol
Catalog No.:BCN6148
CAS No.:127-22-0
- Pimaric acid
Catalog No.:BCN6149
CAS No.:127-27-5
- Lasiocarpine N-oxide
Catalog No.:BCN2002
CAS No.:127-30-0
- Lutein
Catalog No.:BCN6151
CAS No.:127-40-2
- Vitamin A Acetate
Catalog No.:BCC4748
CAS No.:127-47-9
- 2,2-Bis(4-hydroxy-3-isopropylphenyl)propane
Catalog No.:BCC8494
CAS No.:127-54-8
- Sulfacetamide Sodium
Catalog No.:BCC4383
CAS No.:127-56-0
- Sulfamerazine sodium salt
Catalog No.:BCC5205
CAS No.:127-58-2
- Sulfisoxazole
Catalog No.:BCC4860
CAS No.:127-69-5
- Sulfamerazine
Catalog No.:BCC4854
CAS No.:127-79-7
An ontogenetic analysis of locustatachykinin-like expression in the central complex of the grasshopper Schistocerca gregaria.[Pubmed:18635396]
Arthropod Struct Dev. 2008 Nov;37(6):480-91.
We have investigated the ontogenetic basis of locustatachykinin-like expression in a group of cells located in the pars intercerebralis of the grasshopper midbrain. These cells project fibers to the protocerebral bridge and the central body via a characteristic set of fiber bundles called the w, x, y, z tracts. Lineage analyses associate the immunoreactive cells with one of four neuroblasts (termed W, X, Y, Z) in each protocerebral hemisphere of the early embryo. Locustatachykinin Is a ubiquitous myotropic peptide among the insects and its expression in the pars intercerebralis begins at approximately 60-65% of embryogenesis. This coincides with the appearance of the columnar neuroarchitecture characteristic of the central body. The number of immunoreactive cells in a given lineage is initially small, increases significantly in later embryogenesis, and attains the adult situation (about 7% of a lineage) in the first larval instar after hatching. Although each neuroblast generates progeny displaying a spectrum of cell body sizes, there is a clear morphological gradient, which reflects birth order within the lineage. Locustatachykinin expressing cells are located stereotypically at or near the tip of their lineage, which an age profile reveals places them amongst the first born progeny of their respective neuroblasts. Although these neuroblasts begin to generate progeny at approximately 25-27% of embryogenesis, their daughter cells remain quiescent with respect to locustatachykinin expression for over 30% of embryogenesis.
Locustatachykinin isoforms in the locust: distribution and quantification in the central nervous system and action on the oviduct muscle.[Pubmed:10477124]
Peptides. 1999;20(6):687-94.
The presence of locustatachykinin (LomTK)-like immunoreactivity is demonstrated in the central nervous system (CNS) of Locusta migratoria with the use of a polyclonal antiserum raised against LomTK1. By developing a radioimmunoassay with the same antiserum, we have demonstrated picomolar amounts of LomTK-like material in the tissues of the central nervous system. In contrast, only femptomolar amounts of LomTK-like material are associated with the oviduct tissue. The relative amounts of the different LomTK isoforms in the brain and the abdominal ganglionic chain were examined by separating the native peptides on high-performance liquid chromatography and comparing their retention times to synthetic LomTK standards. The amounts of the different isoforms of LomTK differed between and within the two regions of the central nervous system. However, the ratios of the different isoform amounts were similar between the two regions. The myostimulatory activities of LomTKs 1 to 4 were characterized by using the locust oviduct bioassay. LomTKs 1, 2, and 3 appeared to be more efficacious than LomTK4.
The distribution and myotropic activity of locustatachykinin-like peptides in locust midgut.[Pubmed:10573287]
Peptides. 1999;20(10):1159-67.
The midgut of the African migratory locust, Locusta migratoria, was found to contain endocrine-like cells that stained positively for Locustatachykinin I (Lom TK I)-like immunoreactivity. These cells were distributed in an unequal manner throughout the midgut of the locust, with a greater density of Lom TK I-like immunoreactive endocrine-like cells occurring in the posterior region of the midgut. These singly occurring cells appear elongate with an apical extension projecting toward the midgut lumen and a smaller projection extending towards the midgut basal lamina. No immunoreactive neuronal processes were detected along the midgut wall. Radioimmunoassays revealed that the female midgut contained two to three times more Lom TK I-like material than the male midgut, and radioimmunoassay coupled to high-performance liquid chromatography analysis revealed that at least five Locustatachykinin Isoforms appear to be present in the midgut. This distribution of Lom TK I-like material suggests possible functional differences in the various regions of the midgut. The role that these cells may play in locust midgut secretory activity and motility remains unknown. However, the addition of synthetic Lom TK I through IV to a ring type midgut muscle preparation stimulated contraction of midgut circular muscles, suggesting a possible physiological role for these peptides. Dose-response curves constructed for Lom TK I-IV revealed that the peptide-induced contractions increased in a dose-dependent manner.
Diuretic action of the peptide locustatachykinin I: cellular localisation and effects on fluid secretion in Malpighian tubules of locusts.[Pubmed:14706536]
Peptides. 2003 Oct;24(10):1571-9.
In insects primary urine is produced by the Malpighian tubules under hormonal control. Here we have analysed the effects of the peptide Locustatachykinin I (Lom-TK-I) on secretion in isolated Malphigian tubules. We also mapped the distribution of Lom-TK immunoreactivity in the gut in comparison with Locusta diuretic hormone (Lom-DH) and serotonin, two other factors that are active on locust tubules. Lom-TK-I produces an immediate, potent and long-lasting stimulation of fluid secretion. Furthermore, we show that Lom-TK-I acts synergistically with Lom-DH on fluid secretion and demonstrate that Lom-TKs are co-localised with Lom-DH in endocrine cells of the midgut ampullae. Thus, the two peptides might be released together to act synergistically on fluid secretion. Also serotonin and Lom-DH act synergistically and we can demonstrate a plexus of serotonin-containing axon processes over the midgut.
Inactivation of a tachykinin-related peptide: identification of four neuropeptide-degrading enzymes in neuronal membranes of insects from four different orders.[Pubmed:11897392]
Peptides. 2002 Apr;23(4):725-33.
Tachykinin-related peptides (TRP) are widely distributed in the CNS of insects, where they are likely to function as transmitters/modulators. Metabolic inactivation by membrane ecto-peptidases is one mechanism by which peptide signalling is terminated in the CNS. Using locustatachykinin-1 (LomTK-1, GPSGFYGVRamide) as a substrate and several selective peptidase inhibitors, we have compared the types of membrane associated peptidases present in the CNS of four insects, Locusta migratoria, Leucophaea maderae, Drosophila melanogaster and Lacanobia oleracea. A neprilysin (NEP)-like activity cleaving the G-F peptide bond was the major LomTK-1-degrading peptidase detected in locust brain membranes. NEP activity was also found in Leucophaea brain membranes, but the major peptidase was an angiotensin converting enzyme (ACE), cleaving the G-V peptide bond. Drosophila adult head and larval neuronal membranes cleaved the G-F and G-V peptide bonds. Phosphoramidon inhibited both these cleavages, but with markedly different potencies, indicating the presence in the fly brain of two NEP-like enzymes with different substrate and inhibitor specificity. In Drosophila, membrane ACE did not make a significant contribution to the cleavage of the G-V bond. In contrast, ACE was an important membrane peptidase in Lacanobia brain, whereas very little neuronal NEP could be detected. A dipeptidyl peptidase IV (DPP IV) that removed the GP dipeptide from the N-terminus of LomTK-1 was also found in Lacanobia neuronal membranes. This peptidase was a minor contributor to LomTK-1 metabolism by neuronal membranes from all four insect species. In Lacanobia, LomTK-1 was also a substrate for a deamidase that converted LomTK-1 to the free acid form. However, the deamidase was not an integral membrane protein and could be a lysosomal contaminant. It appears that insects from different orders can have different complements of neuropeptide-degrading enzymes. NEP, ACE and the deamidase are likely to be more efficient than the common DPP IV activity at terminating neuropeptide signalling since they cleave close to the C-terminus of the tachykinin, a region essential for maintaining biological activity.
Characterization of a receptor for insect tachykinin-like peptide agonists by functional expression in a stable Drosophila Schneider 2 cell line.[Pubmed:10800964]
J Neurochem. 2000 May;74(5):2182-9.
STKR is an insect G protein-coupled receptor, cloned from the stable fly Stomoxys calcitrans. It displays sequence similarity to vertebrate tachykinin [or neurokinin (NK)] receptors. Functional expression of the cloned STKR cDNA was obtained in cultured Drosophila melanogaster Schneider 2 (S2) cells. Insect tachykinin-like peptides or "insectatachykinins," such as Locusta tachykinin (Lom-TK) III, produced dose-dependent calcium responses in stably transfected S2-STKR cells. Vertebrate tachykinins (or neurokinins) did not evoke any effect at concentrations up to 10(-5) M, but an antagonist of mammalian neurokinin receptors, spantide II, inhibited the Lom-TK III-induced calcium response. Further analysis showed that the agonist-induced intracellular release of calcium ions was not affected by pretreatment of the cells with pertussis toxin. The calcium rise was blocked by the phospholipase C inhibitor U73122. In addition, Lom-TK III was shown to have a stimulatory effect on the accumulation of both inositol 1,4,5-trisphosphate and cyclic AMP. These are the same second messengers that are induced in mammalian neurokinin-dependent signaling processes.


