LoxoprofenCAS# 68767-14-6 |
- Atorvastatin Calcium
Catalog No.:BCC2319
CAS No.:134523-03-8
- Pitavastatin Calcium
Catalog No.:BCC3842
CAS No.:147526-32-7
- Pravastatin sodium
Catalog No.:BCC2321
CAS No.:81131-70-6
- Fluvastatin
Catalog No.:BCC1579
CAS No.:93957-54-1
- Fluvastatin Sodium
Catalog No.:BCC2317
CAS No.:93957-55-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 68767-14-6 | SDF | Download SDF |
| PubChem ID | 3965 | Appearance | Powder |
| Formula | C15H18O3 | M.Wt | 246.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (406.01 mM) *"≥" means soluble, but saturation unknown. | ||
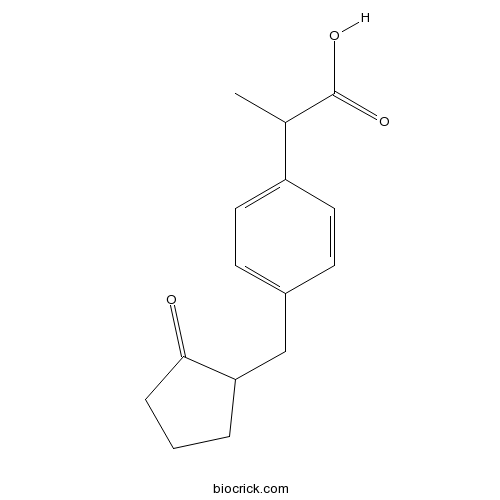
| Chemical Name | 2-[4-[(2-oxocyclopentyl)methyl]phenyl]propanoic acid | ||
| SMILES | CC(C1=CC=C(C=C1)CC2CCCC2=O)C(=O)O | ||
| Standard InChIKey | YMBXTVYHTMGZDW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H18O3/c1-10(15(17)18)12-7-5-11(6-8-12)9-13-3-2-4-14(13)16/h5-8,10,13H,2-4,9H2,1H3,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Loxoprofen is a non-steroidal anti-inflammatory drug.
Target: COX
Loxoprofen is a non-steroidal anti-inflammatory drug in the propionic acid derivatives group, which also includes ibuprofen and naproxen among others. Loxoprofen is a non-selective cyclooxygenase inhibitor, and works by reducing the synthesis of prostaglandins from arachidonic acid. References: | |||||

Loxoprofen Dilution Calculator

Loxoprofen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | 81.2018 mL | 101.5022 mL |
| 5 mM | 0.812 mL | 4.0601 mL | 8.1202 mL | 16.2404 mL | 20.3004 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0601 mL | 8.1202 mL | 10.1502 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Loxoprofen is a non-steroidal anti-inflammatory drug.
- (±)-Pinocembrin
Catalog No.:BCN3537
CAS No.:68745-38-0
- Vellosimine
Catalog No.:BCN4758
CAS No.:6874-98-2
- Arborine
Catalog No.:BCN7480
CAS No.:6873-15-0
- Phellodendrine
Catalog No.:BCN5933
CAS No.:6873-13-8
- Epiberberine
Catalog No.:BCN5387
CAS No.:6873-09-2
- Xanthoplanine
Catalog No.:BCN4246
CAS No.:6872-88-4
- Arteanoflavone
Catalog No.:BCN6824
CAS No.:68710-17-8
- (-)-Lotusine
Catalog No.:BCN8443
CAS No.:6871-67-6
- Echitamine
Catalog No.:BCN4245
CAS No.:6871-44-9
- Asimilobine
Catalog No.:BCN7076
CAS No.:6871-21-2
- 13,18-Dehydroglaucarubinone
Catalog No.:BCN7957
CAS No.:68703-94-6
- 11β,17α-Dihydroxy-6α-methylpregna-1,4-diene-3,20-dione
Catalog No.:BCC8434
CAS No.:6870-94-6
- Corynoxine
Catalog No.:BCN2364
CAS No.:6877-32-3
- 12-Hydroxy-2,3-dihydroeuparin
Catalog No.:BCN8115
CAS No.:68776-42-1
- 1beta-Hydroxyalantolactone
Catalog No.:BCN3508
CAS No.:68776-47-6
- Triclabendazole
Catalog No.:BCC4742
CAS No.:68786-66-3
- Tuberstemonine
Catalog No.:BCN4986
CAS No.:6879-01-2
- Dehydroeburicoic acid
Catalog No.:BCN3646
CAS No.:6879-05-6
- Dipotassium glycyrrhizinate
Catalog No.:BCN8487
CAS No.:68797-35-3
- 4-Oxobedfordiaic acid
Catalog No.:BCN4247
CAS No.:68799-38-2
- 6-Acetyl-2,2-dimethylchroman-4-one
Catalog No.:BCN4248
CAS No.:68799-41-7
- Norfluorocurarine
Catalog No.:BCN4811
CAS No.:6880-54-2
- Dihydrochelerythrine
Catalog No.:BCN2273
CAS No.:6880-91-7
- Sophoridine
Catalog No.:BCN4249
CAS No.:6882-68-4
Preparation and Optimization of Immediate Release/Sustained Release Bilayered Tablets of Loxoprofen Using Box-Behnken Design.[Pubmed:27401334]
AAPS PharmSciTech. 2017 May;18(4):1125-1134.
The aim of our current study was to characterize and optimize Loxoprofen immediate release (IR)/sustained release (SR) tablet utilizing a three-factor, three-level Box-Behnken design (BBD) combined with a desirability function. The independent factors included ratio of drug in the IR layer to total drug (X 1), ratio of HPMC to drug in the SR layer (X 2), and ratio of Eudragit RL PO to drug in the SR layer (X 3). The dependent variables assessed were % drug released in distilled water at 30 min (Y 1), % drug released in pH 1.2 at 2 h (Y 2), and % drug released in pH 6.8 at 12 h (Y 3). The responses were fitted to suitable models and statistical validation was performed using analysis of variance. In addition, response surface graphs and contour plots were constructed to determine the effects of different factor level combinations on the responses. The optimized Loxoprofen IR/SR tablets were successfully prepared with the determined amounts of ingredients that showed close agreement in the predicted and experimental values of tablet characterization and drug dissolution profile. Therefore, BBD can be utilized for successful optimization of Loxoprofen IR/SR tablet, which can be regarded as a suitable substitute for the current marketed formulations.
Characterization of loxoprofen transport in Caco-2 cells: the involvement of a proton-dependent transport system in the intestinal transport of loxoprofen.[Pubmed:27514365]
Biopharm Drug Dispos. 2016 Nov;37(8):447-455.
Loxoprofen, a propionate non-steroidal anti-inflammatory drug (NSAID), is used widely in East Asian countries. However, little is known about the transport mechanisms contributing to its intestinal absorption. The objectives of this study were to characterize the intestinal transport of Loxoprofen using the human intestinal Caco-2 cell model. The transport of Loxoprofen was investigated in cellular uptake studies. The uptake of Loxoprofen into Caco-2 cells was pH- and concentration-dependent, and was described by a Michaelis-Menten equation with passive diffusion (Km : 4.8 mm, Vmax : 142 nmol/mg protein/30 s, and Kd : 2.2 mul/mg protein/30 s). Moreover, the uptake of Loxoprofen was inhibited by a typical monocarboxylate transporter (MCT) inhibitor as well as by various monocarboxylates. The uptake of [(14) C] l-lactic acid, a typical MCT substrate, in Caco-2 cells was saturable with relatively high affinity for MCT. Because Loxoprofen inhibited the uptake of [(14) C] l-lactic acid in a noncompetitive manner, it was unlikely that Loxoprofen uptake was mediated by high-affinity MCT(s). Our results suggest that transport of Loxoprofen in Caco-2 cells is, at least in part, mediated by a proton-dependent transport system. Copyright (c) 2016 John Wiley & Sons, Ltd.
Loxoprofen: A Review in Pain and Inflammation.[Pubmed:27444038]
Clin Drug Investig. 2016 Sep;36(9):771-781.
Loxoprofen (Loxonin((R)), Loxonin((R)) Pap, Loxonin((R)) Tape) is a prodrug-type NSAID that is available in several formulations, including 60 mg tablets, 100 mg hydrogel patches and 50 or 100 mg tape. In active comparator-controlled trials, oral Loxoprofen therapy (ranging from 2 days to 6 weeks' duration depending on the pain type) provided analgesic efficacy that generally did not significantly differ from that of celecoxib for postoperative pain or frozen shoulder, ibuprofen for knee osteoarthritis or naproxen for lumbar pain. In double-blind, double-dummy, multicentre trials, Loxoprofen hydrogel patches were noninferior to oral Loxoprofen with regard to rates of final overall symptomatic improvement over 1-4 weeks in patients with knee osteoarthritis, myalgia or trauma-induced swelling and pain. Loxoprofen hydrogel patches were also noninferior to other commercially available patches (ketoprofen and indometacin) over 2 or 4 weeks in patients with knee osteoarthritis or myalgia in open-label studies. Oral and topical Loxoprofen were generally well tolerated in clinical trials. Thus, Loxoprofen is a useful analgesic option for patients with pain and inflammation, with topical Loxoprofen potentially reducing the risk of gastrointestinal, cardiovascular and renal complications associated with oral NSAID use.
Identification and Structure Elucidation of Forced Degradation Products of the Novel Propionic acid Derivative Loxoprofen: Development of Stability-Indicating Chromatographic Methods Validated as per ICH Guidelines.[Pubmed:27988488]
J Chromatogr Sci. 2017 Apr 1;55(4):417-428.
Loxoprofen sodium (LOX) is a recently developed novel propionic acid derivative. Owing to its instability under both hydrolytic and oxidative conditions, the development of simple, rapid and sensitive methods for its determination in the presence of its possible forced degradation products becomes essential. Two simple chromatographic methods, high-performance thin layer chromatography (HPTLC) and high-performance liquid chromatography (HPLC), were developed associated with ultraviolet (UV) detection. In HPTLC-densitometric method, the separation of LOX from its degradation products was achieved using silica gel F254 plates and toluene:acetone:acetic acid (1.8:1.0:0.1, v/v/v) as the developing system followed by densitometric scanning at 220 nm. In the HPLC-UV method, the separation was performed using isocratic elution system with acetonitrile: 0.15% triethylamine (pH 2.2) (50:50, v/v) on C18 analytical column. The flow rate was optimized at 1.0 mL.min-1 and UV detection was achieved at 220 nm. Validation was performed in accordance with the International Conference on Harmonization guidelines and the method was perfectly applied for determination of LOX in its pharmaceutical preparation. The results obtained were statistically compared to those obtained after application of the official HPLC method, where no significant difference was found incompliance with precision and accuracy. Identification and characterization of the possible hydrolytic degradation product under alkaline conditions and that produced during oxidative degradation using hydrogen peroxide were structurally elucidated using infrared and mass spectrometry analyses.


