MRS 2179 tetrasodium saltSelective P2Y1 antagonist CAS# 1454889-37-2 |
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1454889-37-2 | SDF | Download SDF |
| PubChem ID | 90479745 | Appearance | Powder |
| Formula | C11H13N5O9P2Na4 | M.Wt | 513.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
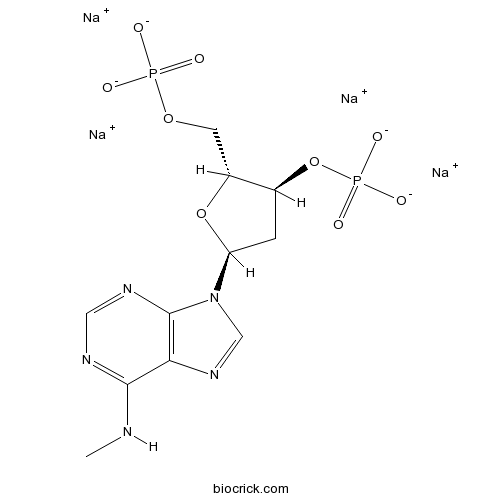
| Chemical Name | tetrasodium;[(2R,3S,5S)-5-[6-(methylamino)purin-9-yl]-2-(phosphonatooxymethyl)oxolan-3-yl] phosphate | ||
| SMILES | CNC1=NC=NC2=C1N=CN2C3CC(C(O3)COP(=O)([O-])[O-])OP(=O)([O-])[O-].[Na+].[Na+].[Na+].[Na+] | ||
| Standard InChIKey | XLPQPYQWGFCKEY-IDAKGYGSSA-J | ||
| Standard InChI | InChI=1S/C11H17N5O9P2.4Na/c1-12-10-9-11(14-4-13-10)16(5-15-9)8-2-6(25-27(20,21)22)7(24-8)3-23-26(17,18)19;;;;/h4-8H,2-3H2,1H3,(H,12,13,14)(H2,17,18,19)(H2,20,21,22);;;;/q;4*+1/p-4/t6-,7+,8-;;;;/m0..../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A competitive antagonist at P2Y1 receptors (KB = 100 nM). Selective over P2X1 (IC50 = 1.15 μM), P2X3 (IC50 = 12.9 μM), P2X2, P2X4, P2Y2, P2Y4 and P2Y6 receptors. Inhibits the upregulation of NTPDase1 by ATPγS. |

MRS 2179 tetrasodium salt Dilution Calculator

MRS 2179 tetrasodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9487 mL | 9.7435 mL | 19.4871 mL | 38.9742 mL | 48.7177 mL |
| 5 mM | 0.3897 mL | 1.9487 mL | 3.8974 mL | 7.7948 mL | 9.7435 mL |
| 10 mM | 0.1949 mL | 0.9744 mL | 1.9487 mL | 3.8974 mL | 4.8718 mL |
| 50 mM | 0.039 mL | 0.1949 mL | 0.3897 mL | 0.7795 mL | 0.9744 mL |
| 100 mM | 0.0195 mL | 0.0974 mL | 0.1949 mL | 0.3897 mL | 0.4872 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PF-06463922
Catalog No.:BCC5568
CAS No.:1454846-35-5
- 4-Benzoylpyridine
Catalog No.:BCC8697
CAS No.:14548-46-0
- LY3009120
Catalog No.:BCC3985
CAS No.:1454682-72-4
- Furano(2'',3'',7,6)-4'-hydroxyflavanone
Catalog No.:BCN6405
CAS No.:1454619-70-5
- PU-WS13
Catalog No.:BCC6425
CAS No.:1454619-14-7
- Isolintetralin
Catalog No.:BCN3052
CAS No.:145459-30-9
- Heterophyllin B
Catalog No.:BCN2768
CAS No.:145459-19-4
- L-692,585
Catalog No.:BCC7305
CAS No.:145455-35-2
- Homalomenol A
Catalog No.:BCN1647
CAS No.:145400-03-9
- 1,4,7-Eudesmanetriol
Catalog No.:BCN1646
CAS No.:145400-02-8
- GDC-0994
Catalog No.:BCC6371
CAS No.:1453848-26-4
- Sideroxylonal A
Catalog No.:BCN1645
CAS No.:145382-68-9
- (2R,3S)-Boc-3-Phenylisoserine
Catalog No.:BCN8362
CAS No.:145514-62-1
- 1,7-Dimethoxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7539
CAS No.:145523-71-3
- Mitiglinide Calcium
Catalog No.:BCC5000
CAS No.:145525-41-3
- Eucamalol
Catalog No.:BCN1648
CAS No.:145544-91-8
- Sophocarpine
Catalog No.:BCN5971
CAS No.:145572-44-7
- Cyclocommunol
Catalog No.:BCN3375
CAS No.:145643-96-5
- NNC 711
Catalog No.:BCC7176
CAS No.:145645-62-1
- 2'-O-Benzoylpaeoniflorin
Catalog No.:BCN7803
CAS No.:1456598-64-3
- SH-4-54
Catalog No.:BCC5483
CAS No.:1456632-40-8
- HG-9-91-01
Catalog No.:BCC4071
CAS No.:1456858-58-4
- 3-Allylrhodanine
Catalog No.:BCC8604
CAS No.:1457-47-2
- AR-C 66096 tetrasodium salt
Catalog No.:BCC6004
CAS No.:145782-74-7
Stimulation of the P2Y1 receptor up-regulates nucleoside-triphosphate diphosphohydrolase-1 in human retinal pigment epithelial cells.[Pubmed:17626796]
J Pharmacol Exp Ther. 2007 Oct;323(1):157-64.
Stimulation of receptors for either ATP or adenosine leads to physiologic changes in retinal pigment epithelial (RPE) cells that may influence their relationship with the adjacent photoreceptors. The ectoenzyme nucleoside-triphosphate diphosphohydrolase-1 (NTPDase1) catalyzes the dual dephosphorylation of ATP and ADP to AMP. Although NTPDase1 can consequently control the balance between ATP and adenosine, it is unclear how its expression and activity are regulated. Classic negative feedback theory predicts an increase in enzyme activity in response to enhanced exposure to substrate. This study asked whether exposure to ATP increases NTPDase1 activity in RPE cells. Although levels of NTPDase1 mRNA and protein in cultured human ARPE-19 cells were generally low under control conditions, exposure to slowly hydrolyzable ATPgammaS led to a time-dependent increase in NTPDase1 mRNA that was accompanied by a rise in levels of the functional 78-kDa protein. Neither NTPDase2 nor NTPDase3 mRNA message was elevated by ATPgammaS. The ATPase activity of cells increased in parallel, indicating the up-regulation of NTPDase1 was functionally relevant. The up-regulation of NTPDase1 protein was partially blocked by P2Y1 receptor inhibitors MRS2179 (N6-methyl-2'-deoxyadenosine-3',5'-bisphosphate) and MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2'-deoxyadenosine 3',5'-bisphosphate] and increased by P2Y1 receptor agonist MRS2365 [(N)-methanocarba-2MeSADP]. In conclusion, prolonged exposure to extracellular ATPgammaS increased NTPDase1 message and protein levels and increased ecto-ATPase activity. This up-regulation reflects a feedback circuit, mediated at least in part by the P2Y1 receptor, to regulate levels of extracellular purines in subretinal space. NTPDase1 levels may thus serve as an index for increased extracellular ATP levels under certain pathologic conditions, although other mechanisms could also contribute.
Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y(1) receptor ligands.[Pubmed:10715151]
J Med Chem. 2000 Mar 9;43(5):829-42.
The structure-activity relationships of adenosine-3', 5'-bisphosphates as P2Y(1) receptor antagonists have been explored, revealing the potency-enhancing effects of the N(6)-methyl group and the ability to substitute the ribose moiety (Nandanan et al. J. Med. Chem. 1999, 42, 1625-1638). We have introduced constrained carbocyclic rings (to explore the role of sugar puckering), non-glycosyl bonds to the adenine moiety, and a phosphate group shift. The biological activity of each analogue at P2Y(1) receptors was characterized by measuring its capacity to stimulate phospholipase C in turkey erythrocyte membranes (agonist effect) and to inhibit its stimulation elicited by 30 nM 2-methylthioadenosine-5'-diphosphate (antagonist effect). Addition of the N(6)-methyl group in several cases converted pure agonists to antagonists. A carbocyclic N(6)-methyl-2'-deoxyadenosine bisphosphate analogue was a pure P2Y(1) receptor antagonist and equipotent to the ribose analogue (MRS 2179). In the series of ring-constrained methanocarba derivatives where a fused cyclopropane moiety constrained the pseudosugar ring of the nucleoside to either a Northern (N) or Southern (S) conformation, as defined in the pseudorotational cycle, the 6-NH(2) (N)-analogue was a pure agonist of EC(50) 155 nM and 86-fold more potent than the corresponding (S)-isomer. The 2-chloro-N(6)-methyl-(N)-methanocarba analogue was an antagonist of IC(50) 51.6 nM. Thus, the ribose ring (N)-conformation appeared to be favored in recognition at P2Y(1) receptors. A cyclobutyl analogue was an antagonist with IC(50) of 805 nM, while morpholine ring-containing analogues were nearly inactive. Anhydrohexitol ring-modified bisphosphate derivatives displayed micromolar potency as agonists (6-NH(2)) or antagonists (N(6)-methyl). A molecular model of the energy-minimized structures of the potent antagonists suggested that the two phosphate groups may occupy common regions. The (N)- and (S)-methanocarba agonist analogues were docked into the putative binding site of the previously reported P2Y(1) receptor model.
Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2'-deoxyadenosine 3',5'-bisphosphate.[Pubmed:9630335]
Br J Pharmacol. 1998 May;124(1):1-3.
The antagonist activity of N6-methyl 2'-deoxyadenosine 3',5'-bisphosphate (N6MABP) has been examined at the phospholipase C-coupled P2Y1 receptor of turkey erythrocyte membranes. N6MABP antagonized 2MeSATP-stimulated inositol phosphate hydrolysis with a potency approximately 20 fold greater than the previously studied parent molecule, adenosine 3',5'-bisphosphate. The P2Y1 receptor antagonism observed with N6MABP was competitive as revealed by Schild analysis (pK(B) = 6.99 +/- 0.13). Whereas N6MABP was an antagonist at the human P2Y1 receptor, no antagonist effect of N6MABP was observed at the human P2Y2, human P2Y4 or rat P2Y6 receptors.


