Meclofenamate SodiumCAS# 6385-02-0 |
- Licofelone
Catalog No.:BCC4432
CAS No.:156897-06-2
- Lumiracoxib
Catalog No.:BCC4440
CAS No.:220991-20-8
- Bufexamac
Catalog No.:BCC4427
CAS No.:2438-72-4
- Triflusal
Catalog No.:BCC4443
CAS No.:322-79-2
- Nabumetone
Catalog No.:BCC4434
CAS No.:42924-53-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 6385-02-0 | SDF | Download SDF |
| PubChem ID | 4038 | Appearance | Powder |
| Formula | C14H10Cl2NNaO2 | M.Wt | 318.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 250 mg/mL (785.84 mM; Need ultrasonic) | ||
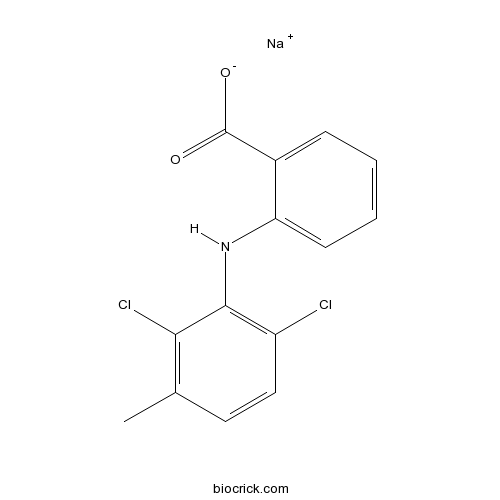
| Chemical Name | sodium;2-(2,6-dichloro-3-methylanilino)benzoate | ||
| SMILES | CC1=C(C(=C(C=C1)Cl)NC2=CC=CC=C2C(=O)[O-])Cl.[Na+] | ||
| Standard InChIKey | OGPIIGMUPMPMNT-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C14H11Cl2NO2.Na/c1-8-6-7-10(15)13(12(8)16)17-11-5-3-2-4-9(11)14(18)19;/h2-7,17H,1H3,(H,18,19);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Meclofenamate Sodium Dilution Calculator

Meclofenamate Sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1434 mL | 15.7168 mL | 31.4337 mL | 62.8674 mL | 78.5842 mL |
| 5 mM | 0.6287 mL | 3.1434 mL | 6.2867 mL | 12.5735 mL | 15.7168 mL |
| 10 mM | 0.3143 mL | 1.5717 mL | 3.1434 mL | 6.2867 mL | 7.8584 mL |
| 50 mM | 0.0629 mL | 0.3143 mL | 0.6287 mL | 1.2573 mL | 1.5717 mL |
| 100 mM | 0.0314 mL | 0.1572 mL | 0.3143 mL | 0.6287 mL | 0.7858 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NMDA (N-Methyl-D-aspartic acid)
Catalog No.:BCC4590
CAS No.:6384-92-5
- H-Glu-OMe
Catalog No.:BCC2924
CAS No.:6384-08-3
- Phyllostadimer A
Catalog No.:BCN4178
CAS No.:638203-32-4
- Z-D-Arg-OH
Catalog No.:BCC3575
CAS No.:6382-93-0
- 5-(3-Chlorophenyl)-N-[4-(morpholin-4-ylmethyl)phenyl]furan-2-carboxamide
Catalog No.:BCC3636
CAS No.:638156-11-3
- Dihydroalpinumisoflavone
Catalog No.:BCN4177
CAS No.:63807-90-9
- Erythrinin A
Catalog No.:BCN3203
CAS No.:63807-86-3
- Erythrinin C
Catalog No.:BCN4176
CAS No.:63807-85-2
- 2-Amino-3-dodecanol
Catalog No.:BCN4175
CAS No.:
- beta-Amyrone
Catalog No.:BCN4179
CAS No.:638-97-1
- alpha-Amyrin
Catalog No.:BCN3341
CAS No.:638-95-9
- Desonide
Catalog No.:BCC4967
CAS No.:638-94-8
- Cloxyfonac
Catalog No.:BCC5473
CAS No.:6386-63-6
- Gypsogenin
Catalog No.:BCC8993
CAS No.:639-14-5
- Ajmalidine
Catalog No.:BCN3491
CAS No.:639-30-5
- Akuammidine
Catalog No.:BCN6509
CAS No.:639-36-1
- Pinoresinol diglucoside
Catalog No.:BCN1093
CAS No.:63902-38-5
- Zinc Phytate
Catalog No.:BCN8302
CAS No.:63903-51-5
- LH846
Catalog No.:BCC4246
CAS No.:639052-78-1
- VUF 5681 dihydrobromide
Catalog No.:BCC7383
CAS No.:639089-06-8
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- (3R,10S)-Heptadeca-1,8-diene-4,6-diyne-3,10-diol
Catalog No.:BCC9111
CAS No.:63910-76-9
- Artemisinin
Catalog No.:BCN5814
CAS No.:63968-64-9
- Hopeyhopin
Catalog No.:BCN7533
CAS No.:63975-56-4
Comparison of the kinetics of sodium meclofenamate versus meclofenamic acid after oral administration to sheep.[Pubmed:8578896]
Zentralbl Veterinarmed A. 1995 May;42(3):177-83.
Meclofenamates are non-steroidal anti-inflammatory agents used in ruminants for the prevention and the treatment of anaphylactic processes. The objective of the present work was to study possible kinetic variations due to the chemical form of meclofenamates administered by the oral route to adult sheep. Six Rubia del Molar female sheep (2-3 years old, 47-57 kg) were used. Initially, an intravenous administration of sodium meclofenamate (2.2 mg/kg bwt) was given; the obtained kinetic results were in agreement with data from other authors. Oral administrations (20 mg/kg bwt) of sodium meclofenamate and meclofenamic acid were then given. When the reticular groove was opened, both drug forms showed a single meclofenamate plasma peak; t2max were 60.0 +/- 10.61 min and 127.50 +/- 22.5 min for the sodium and acid form, respectively. The elimination rate constants (beta) were not significantly different, but the absorption half-lives were (14.69 +/- 3.21 min for the sodium form and 61.07 +/- 21.7 min for the acid form). The bioavailability was 48.6 +/- 4.3% for sodium meclofenamate and 65.1 +/- 2.8% for meclofenamic acid. Thus, the chemical form (sodium versus acid) alters the oral bioavailability and tmax of meclofenamates in adult sheep. These findings agree with the behaviour of meclofenamates in man.
Efficacy of meclofenamate sodium (Meclomen) in the treatment of rapidly progressive periodontitis.[Pubmed:8227450]
J Clin Periodontol. 1993 Oct;20(9):635-40.
This 6-month, double-blind, controlled clinical trial determined the efficacy of the non-steroidal anti-inflammatory drug, Meclofenamate Sodium (Meclomen), as an adjunct to scaling and root planing in the treatment of rapidly progressive periodontitis (RPP). 22 subjects (7 male, 15 female) aged 36.5 +/- 7.88 years with RPP and disease-active sites as determined by pretreatment bone scan had standardized radiographs at baseline and 6 months, and clinical measurements at baseline, 3 and 6 months. Following full-mouth scaling and root planing, subjects were randomly assigned to either a placebo, 50 or 100 mg Meclofenamate Sodium bid group. Bone change over the 6-month period as assessed by subtraction radiography was the primary efficacy determinant. Specialized software was used to isolate the lesion from the subtraction image and to measure bone change along the root surface. ANOVA using the subject as the unit of analysis revealed a significant dose response (p < 0.001) with the placebo group having a mean bone loss of 0.42 +/- 0.06 mm and the low and high dose groups having mean bone gains of 0.07 +/- 0.05 and 0.20 +/- 0.07 mm, respectively. These findings indicate that Meclofenamate Sodium may be a useful adjunct in the treatment of rapidly progressive periodontitis.
Single dose and multidose analgesic study of ibuprofen and meclofenamate sodium after third molar surgery.[Pubmed:8284070]
Oral Surg Oral Med Oral Pathol. 1993 Dec;76(6):680-7.
The purpose of this study was to compare the analgesic efficacy and safety of Meclofenamate Sodium with ibuprofen after dental impaction surgery. This study was double-blind and used a unique methodology. Patients (N = 254) were first randomized into the single dose phase of the study that included placebo, meclofenamate 50 mg, meclofenamate 100 mg, ibuprofen 200 mg, and ibuprofen 400 mg, followed by a 7-day multidose phase in which patients in the placebo group were rerandomized into one of the active treatment cells. In the single dose phase, all active treatments were significantly more efficacious than placebo for every summary analgesic measure. A positive dose-response was seen for both active drugs with meclofenamate 100 mg and ibuprofen 400 mg exhibiting the greatest efficacy for pain relief, pain reduction, time to remedication, and overall evaluation. Side effects were reported by 26 patients. They were evenly distributed among treatment groups with headache and drowsiness being the most common. During the multidose phase, there were only small differences in efficacy measures among active treatment groups. However, meclofenamate produced a higher incidence of stomach cramps and diarrhea than did ibuprofen (8.8% and 7.2% versus 0.8% and 0.8%). This study indicates that higher doses of nonsteroidal anti-inflammatory drugs are most effective immediately after surgery and that lower doses of these drugs can be used after the first postoperative day. The side effect profile of nonsteroidal anti-inflammatory analgesics is best observed with the use of a multidose study design.
Different effects of the nonsteroidal anti-inflammatory drugs meclofenamate sodium and naproxen sodium on proteasome activity in cardiac cells.[Pubmed:27049794]
J Mol Cell Cardiol. 2016 May;94:131-144.
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) like Meclofenamate Sodium (MS), used to reduce pain, has been associated with an increased risk of cardiovascular disease (CVD). Naproxen (NAP), another NSAID, is not associated with increased risk of CVD. The molecular mechanism(s) by which NSAIDs induce CVD is unknown. We investigated the effects of MS and NAP on protein homeostasis and cardiotoxicity in rat cardiac H9c2 cells and murine neonatal cardiomyocytes. MS, but not NAP, significantly inhibited proteasome activity and reduced cardiac cell viability at pharmacological levels found in humans. Although proteasome subunit gene and protein expression were unaffected by NSAIDs, MS treated cell lysates showed higher 20S proteasome content, while purified proteasomes from MS treated cells had lower proteasome activity and higher levels of oxidized subunits than proteasomes from control cells. Addition of exogenous proteasome to MS treated cells improved cell viability. Both MS and NAP increased ROS production, but the rate of ROS production was greater in MS than in NAP treated cells. The ROS production is likely from mitochondria, as MS inhibited mitochondrial Complexes I and III, major sources of ROS, while NAP inhibited Complex I. MS also impaired mitochondrial membrane potential while NAP did not. Antioxidants were able to prevent the reduced cell viability caused by MS treatment. These results suggest that NSAIDs induce cardiotoxicity by a ROS dependent mechanism involving mitochondrial and proteasome dysfunction and may explain why some NSAIDs should not be given to patients for long periods.


