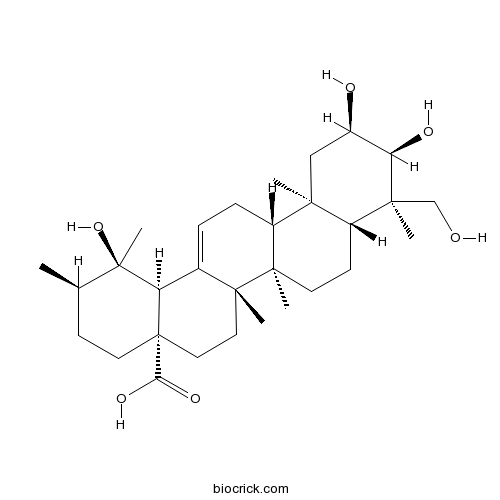
Myrianthic acidCAS# 89786-84-5 |
- 19 alpha-Hydroxyasiatic acid
Catalog No.:BCN8720
CAS No.:70868-78-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 89786-84-5 | SDF | Download SDF |
| PubChem ID | 182497 | Appearance | Powder |
| Formula | C30H48O6 | M.Wt | 504.70 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2R,4aS,6aR,6aS,6bR,8aR,9R,10S,11R,12aR,14bS)-1,10,11-trihydroxy-9-(hydroxymethyl)-1,2,6a,6b,9,12a-hexamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | ||
| SMILES | CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CC(C(C5(C)CO)O)O)C)C)C2C1(C)O)C)C(=O)O | ||
| Standard InChIKey | YCOKATFNRPZIIU-MGBZEVKYSA-N | ||
| Standard InChI | InChI=1S/C30H48O6/c1-17-9-12-30(24(34)35)14-13-27(4)18(22(30)29(17,6)36)7-8-21-25(2)15-19(32)23(33)26(3,16-31)20(25)10-11-28(21,27)5/h7,17,19-23,31-33,36H,8-16H2,1-6H3,(H,34,35)/t17-,19-,20-,21-,22-,23-,25+,26+,27-,28-,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Myrianthic acid shows inhibitory activities on foam cell formation in human monocyte-derived macrophages induced by acetylated low-density lipoproteins at a 50 uM concentration. 2. Myrianthic acid shows anticancer activities. 3. Myrianthic acid is equivalently inhibitive as acetylsalicylic acid (IC50: 57.0 microM) on epinephrine induced platelet aggregation. |
| Targets | AChR | PAFR |

Myrianthic acid Dilution Calculator

Myrianthic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9814 mL | 9.9069 mL | 19.8138 mL | 39.6275 mL | 49.5344 mL |
| 5 mM | 0.3963 mL | 1.9814 mL | 3.9628 mL | 7.9255 mL | 9.9069 mL |
| 10 mM | 0.1981 mL | 0.9907 mL | 1.9814 mL | 3.9628 mL | 4.9534 mL |
| 50 mM | 0.0396 mL | 0.1981 mL | 0.3963 mL | 0.7926 mL | 0.9907 mL |
| 100 mM | 0.0198 mL | 0.0991 mL | 0.1981 mL | 0.3963 mL | 0.4953 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,3,24-Trihydroxy-12-ursen-28-oic acid
Catalog No.:BCN1314
CAS No.:89786-83-4
- Tazobactam acid
Catalog No.:BCC9160
CAS No.:89786-04-9
- Toremifene Citrate
Catalog No.:BCC4487
CAS No.:89778-27-8
- Toremifene
Catalog No.:BCC2010
CAS No.:89778-26-7
- AST-1306
Catalog No.:BCC3727
CAS No.:897383-62-9
- 7-O-Acetyl-4-O-demethylpolysyphorin
Catalog No.:BCN3984
CAS No.:89706-39-8
- KX2-391
Catalog No.:BCC5080
CAS No.:897016-82-9
- Androstadienedione
Catalog No.:BCC8824
CAS No.:897-06-3
- VX-11e
Catalog No.:BCC2051
CAS No.:896720-20-0
- BMH-21
Catalog No.:BCC5580
CAS No.:896705-16-1
- AT9283
Catalog No.:BCC2173
CAS No.:896466-04-9
- trans-3-Oxo-alpha-ionol
Catalog No.:BCN3385
CAS No.:896107-70-3
- Aceclofenac
Catalog No.:BCC5233
CAS No.:89796-99-6
- 5-Ethyltio-1H-Tetrazole
Catalog No.:BCC2844
CAS No.:89797-68-2
- PF-3758309
Catalog No.:BCC1853
CAS No.:898044-15-0
- YS-035 hydrochloride
Catalog No.:BCC6639
CAS No.:89805-39-0
- Esculentoside D
Catalog No.:BCN5013
CAS No.:89808-50-4
- XL228
Catalog No.:BCC2058
CAS No.:898280-07-4
- 4-(4-(5-(Aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one hydrochloride
Catalog No.:BCC8647
CAS No.:898543-06-1
- 3-Epichromolaenide
Catalog No.:BCN7241
CAS No.:89913-53-1
- 3-O-Acetyl-alpha-boswellic acid
Catalog No.:BCN2671
CAS No.:89913-60-0
- Beta-Carboline-1-propanoic acid
Catalog No.:BCN5805
CAS No.:89915-39-9
- Gymnoside III
Catalog No.:BCN8218
CAS No.:899430-03-6
- A 438079 hydrochloride
Catalog No.:BCC1317
CAS No.:899431-18-6
Biological activities of triterpenoids from Poraqueiba sericea stems.[Pubmed:27736194]
Nat Prod Res. 2017 Jun;31(11):1333-1338.
Eleven compounds were isolated from Poraqueiba sericea stems and identified as niga-ichigoside-F1 (1), trachelosperoside B1 (2), 4-epi-niga-ichigoside (7), 19alpha-hydroxyasiatic acid (3), Myrianthic acid (4), hyptatic acid (5), trachelosperogenin B (6), arjunolic acid (8), and trachelosperogenin E (9), secologanoside (10) and secoxyloganin (11). Compounds 1-11 were tested for their antileishmanial activities against Leishmania infantum promastigotes, 1-6 and 8-11 were tested for their cytotoxic activities on fibroblasts, 1-3, 5-6, 8-11 were evaluated for their anti-elastase and anti-acetylcholinesterase assays activities by a spectrophotometric method and 1-2, 5 and 7-10 were tested using bioautography for their beta-glucosidase. No antileishmanial activity was detected; compounds 1, 2 and 11 showed a moderate cytotoxic activity with IC50 17.7, 20.5 and 10.9 mug/mL, respectively; compounds 2, 8, 9 and 10 gave a percentage of inhibition ranging from 13 to 16% (at 50 mug/mL) and compounds 1 and 2 showed an inhibition zone on beta-glucosidase and anti-acetylcholinesterase assays.
Anti-platelet pentacyclic triterpenoids from leaves of Campsis grandiflora.[Pubmed:15180300]
Arch Pharm Res. 2004 Apr;27(4):376-80.
Five pentacyclic triterpenoids, oleanolic acid (1), hederagenin (2), ursolic acid (3), tormentic acid (4) and Myrianthic acid (5), were isolated from the methanol extract of the leaves of Campsis grandiflora, and structures of the compounds were established by the spectroscopic methods. Compounds 2, 3, 4, and 5 were isolated for the first time from the genus Campsis. All of the compounds (IC50: 45.3, 32.8, 82.6, 42.9 and 46.2 microM respectively) were as equivalently inhibitive as acetylsalicylic acid (IC50: 57.0 microM) on epinephrine induced platelet aggregation.
[Triterpenes from herb of Potentilla chinesis].[Pubmed:17285988]
Zhongguo Zhong Yao Za Zhi. 2006 Nov;31(22):1875-9.
OBJECTIVE: To study the chemical constituents of Potentilla chinesis and their anticancer activities. METHOD: Chemical constituents were isolated by repeated column chromatography (Toyopearl HW-40C and preparative HPLC). The structures were elucidated on the basis of spectral data analysis. The isolated compounds were screened with two anticancer models. RESULT: Fifteen triterpenes, alpha-amyrin (1) , beta-amyrin (2) , ursolic acid (3) , corosolic acid (4), euscaphic acid (5) , pomolic acid (6) , tormentic acid (7), 2alpha, 3alpha-dihydroxyurs-12-en-28-oic acid (8), 2beta, 3beta, 19alpha-trihydroxyurs-12-en-28-oic acid (9), asiatic acid (10) , 24-hydroxy tormentic acid (11) , Myrianthic acid (12), oleanolic acid (13), maslinic acid (14) and 2alpha, 3alpha-dihydroxyolean-12-en-28-oic acid (15) , were isolated from P. chinesis. CONCLUSION: Compounds 1, 2, 4 -15 were isolated from the plant for the first time. Compounds 4, 8 - 10, 12, 14 and 15 show anticancer activities. Compounds 4, 9 show strong activities.
Triterpenoids from the fruits and leaves of the blackberry (Rubus allegheniensis) and their inhibitory activities on foam cell formation in human monocyte-derived macrophage.[Pubmed:25033392]
Nat Prod Res. 2014;28(24):2347-50.
From the methanol extract of the fruits of the blackberry (Rubus allegheniensis Port.), four triterpenoids - pomolic acid (1), tormentic acid (2), euscaphic acid (3) and 1beta-hydroxyeuscaphic acid (4) - were isolated, while six triterpenoids - 2, 3, Myrianthic acid (5), ziyu glycoside II (6), sericic acid (7) and 19-hydroxy-2,3-secours-12-ene-2,3,28-trioic acid 3-methyl ester (8) - were obtained from the methanol extract of the leaves of this plant. Their structures were determined on the basis of spectral data. Compounds 1-8 were examined for their inhibitory activities on foam cell formation in human monocyte-derived macrophages induced by acetylated low-density lipoproteins at a 50 muM concentration. Among the tested compounds, 1 showed the strongest activity, with the inhibitory effect being 90%. The inhibitory activities of 2-8 were evaluated to be 30%, 32%, 33%, 4%, 48%, 4% and 24%, respectively. Further, the structure-activity relationship of these compounds was investigated.


