NateglinideInsulin secretagog agent CAS# 105816-04-4 |
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
- MK-5172 sodium salt
Catalog No.:BCC1765
CAS No.:1425038-27-2
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Vaniprevir
Catalog No.:BCC2030
CAS No.:923590-37-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 105816-04-4 | SDF | Download SDF |
| PubChem ID | 443871 | Appearance | Powder |
| Formula | C19H27NO3 | M.Wt | 317.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | A-4166, Senaglinide | ||
| Solubility | DMSO : 100 mg/mL (315.04 mM; Need ultrasonic) | ||
| Chemical Name | (2R)-3-phenyl-2-[[(1S,3S)-3-propan-2-ylcyclohexanecarbonyl]amino]propanoic acid | ||
| SMILES | CC(C)C1CCCC(C1)C(=O)NC(CC2=CC=CC=C2)C(=O)O | ||
| Standard InChIKey | CZCVBLROPSHDRZ-YESZJQIVSA-N | ||
| Standard InChI | InChI=1S/C19H27NO3/c1-13(2)15-9-6-10-16(12-15)18(21)20-17(19(22)23)11-14-7-4-3-5-8-14/h3-5,7-8,13,15-17H,6,9-12H2,1-2H3,(H,20,21)(H,22,23)/t15-,16-,17+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Kir6 (KATP) blocker. Exhibits in vitro tissue selectivity for pancreatic β-cell-type Kir6 channels over cardiovascular Kir6 channels; displays high affinity for SUR1/Kir6.2 channels. Hypoglycemic agent; stimulates insulin secretion from pancreatic β-cells by increasing cytosolic Ca2+ concentration. |

Nateglinide Dilution Calculator

Nateglinide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1504 mL | 15.752 mL | 31.504 mL | 63.008 mL | 78.76 mL |
| 5 mM | 0.6301 mL | 3.1504 mL | 6.3008 mL | 12.6016 mL | 15.752 mL |
| 10 mM | 0.315 mL | 1.5752 mL | 3.1504 mL | 6.3008 mL | 7.876 mL |
| 50 mM | 0.063 mL | 0.315 mL | 0.6301 mL | 1.2602 mL | 1.5752 mL |
| 100 mM | 0.0315 mL | 0.1575 mL | 0.315 mL | 0.6301 mL | 0.7876 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
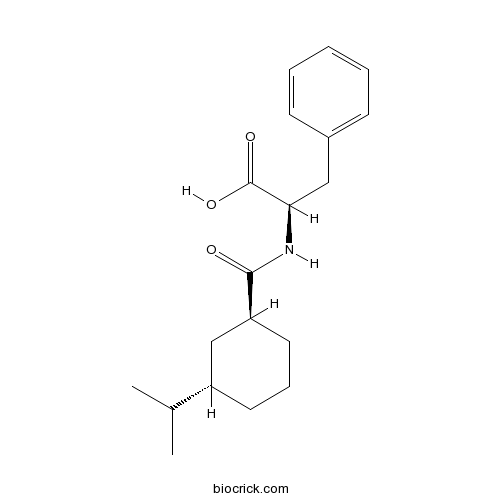
Nateglinide is an insulin secretagog agent used for the treatment of non-insulin-dependent diabetes mellitus (NIDDM).Nateglinide is an oral antihyperglycemic agent used for the treatment of non-insulin-dependent diabetes mellitus (NIDDM). It belongs to th
- E-3810
Catalog No.:BCC1541
CAS No.:1058137-23-7
- Sitostenone
Catalog No.:BCN5868
CAS No.:1058-61-3
- Fmoc-Ser(tBu)-OPfp
Catalog No.:BCC3545
CAS No.:105751-13-1
- Methyl ganoderate C6
Catalog No.:BCN3259
CAS No.:105742-81-2
- Ganoderic acid C6
Catalog No.:BCN3257
CAS No.:105742-76-5
- AL 8697
Catalog No.:BCC8037
CAS No.:1057394-06-5
- Androstanolone 17-benzoate
Catalog No.:BCC8825
CAS No.:1057-07-4
- AT13148
Catalog No.:BCC5360
CAS No.:1056901-62-2
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- CYT387
Catalog No.:BCC2196
CAS No.:1056634-68-4
- Mizolastine dihydrochloride
Catalog No.:BCC4132
CAS No.:1056596-82-7
- ML 9 hydrochloride
Catalog No.:BCC6644
CAS No.:105637-50-1
- Tropisetron Hydrochloride
Catalog No.:BCC4027
CAS No.:105826-92-4
- TSTU
Catalog No.:BCC2828
CAS No.:105832-38-0
- Obtusilin
Catalog No.:BCN2697
CAS No.:105870-59-5
- (tert-Butoxycarbonyl)oxycefcapene pivoxil
Catalog No.:BCC8403
CAS No.:105889-80-3
- Taraxasterol
Catalog No.:BCN5869
CAS No.:1059-14-9
- Doxycycline HCl
Catalog No.:BCC3772
CAS No.:10592-13-9
- STEARDA
Catalog No.:BCC7288
CAS No.:105955-10-0
- OLDA
Catalog No.:BCC7138
CAS No.:105955-11-1
- Clinafloxacin CI96 AM1091
Catalog No.:BCC3754
CAS No.:105956-97-6
- Sulfocostunolide B
Catalog No.:BCN5870
CAS No.:1059671-65-6
- 2-(3,4-Dihydroxyphenyl)ethanol
Catalog No.:BCN5871
CAS No.:10597-60-1
- Geraniol
Catalog No.:BCN2631
CAS No.:106-24-1
Comparison of sitagliptin with nateglinide on postprandial glucose and related hormones in drug-naive Japanese patients with type 2 diabetes mellitus: A pilot study.[Pubmed:26417414]
J Diabetes Investig. 2015 Sep;6(5):560-6.
AIMS/INTRODUCTION: Dipeptidyl peptidase-4 inhibitors and glinides are effective in reducing postprandial hyperglycemia. However, little information is available on the comparative effects of the two drugs on the levels of postprandial glucose. The aim of the present study was to compare the effects of sitagliptin and Nateglinide on meal tolerance tests in drug-naive patients with type 2 diabetes mellitus. MATERIALS AND METHODS: The study participants were 19 patients with type 2 diabetes mellitus, which was inadequately controlled by diet and exercise. An open-label, prospective, cross-over trial was carried out to compare the effects of single-dose sitagliptin and Nateglinide on the postprandial glucose level and its related hormones during meal tests. RESULTS: The change in area under the curve (AUC) of glucose from 0 to 180 min (AUC0-180 min) during the meal test by Nateglinide was similar to that by sitagliptin. As expected, the change in active glucagon like peptide-1 was significantly higher after a single-dose of sitagliptin than Nateglinide. Then, insulin secretion relative to glucose elevation (ISG) (DeltaISG0-180 min: DeltaAUC0-180 min insulin/AUC0-180 min glucose) was significantly enhanced by Nateglinide compared with sitagliptin. Conversely, glucagon level (DeltaAUC0-180 min glucagon) was increased by administration of Nateglinide, whereas the glucagon level was reduced by administration of sitagliptin. CONCLUSIONS: The effects of sitagliptin on postprandial glucose levels were similar to those of Nateglinide in drug-naive type 2 diabetes patients. However, the induced changes in insulin, active glucagon-like peptide-1 and glucagon during meal loading suggest that reduction of postprandial hyperglycemia was achieved by the unique effect of each drug.
Effects of nateglinide and rosiglitazone on pancreatic alpha- and beta-cells, GLP-1 secretion and inflammatory markers in patients with type 2 diabetes: randomized crossover clinical study.[Pubmed:26734075]
Diabetol Metab Syndr. 2016 Jan 4;8:1.
BACKGROUND: To compare the effects of Nateglinide and rosiglitazone on inflammatory markers, GLP-1 levels and metabolic profile in patients with type 2 diabetes (DM2). METHODS: A prospective study was performed in 20 patients with DM2, mean age 51.82 +/- 8.05 years, previously treated with dietary intervention. Participants were randomized into rosiglitazone (4-8 mg/day) or Nateglinide (120 mg 3 times a day) therapy. After 4 months, the patients were crossed-over with 8 weeks washout period to the alternative treatment for an additional 4-month period on similar dosage schedule. The following variables were assessed before and after 4 months of each treatment period: (1) a test with a standardized 500 calories meal for 5 h including frequent measurements of glucose, insulin, glucagon, proinsulin, GLP-1, free fat acids (FFA), and triglycerides levels was obtained. The lipid profile and HbA1 levels were measured at fasting. (2) Haemostatic and inflammatory markers: platelet aggregation, fibrinogen, PAI-1 activity, C reactive protein (CRP), IL-6, TNF-alpha, leptin, sICAM and TGFbeta levels. RESULTS: Both therapy decreased blood glucose levels under the postprandial curve but neither affected glucagon and GLP-1 levels. Nateglinide was associated with higher insulin and pro-insulin secretion, but similar pro-insulin/insulin ratio when compared with rosiglitazone. Only rosiglitazone decreased Homa beta, PAI-1 activity, CRP, fibrinogen, TGFbeta, FFA and triglyceride levels. CONCLUSIONS: Nateglinide and rosiglitazone were effective in improving glucose and lipid profile and beta cell function, but rosiglitazone afforded a better anti-inflammatory effect. No drug restored alpha cell sensitivity or changed GLP-1 levels. Maintenance of haemostatic factors, inflammatory factors and glucagon levels can be related to the continuously worsening of cardiovascular function and glucose control observed in DM2.
Preparation of polyclonal antibodies for nateglinide (NTG) and development of a sensitive chemiluminescent immunoassay to detect NTG in tablets and serum.[Pubmed:26695294]
Talanta. 2016;146:483-9.
In this study, we prepared polyclonal antibodies against anti-diabetic drug Nateglinide (NTG), and established a sensitive chemiluminescent immunoassay (CLIA) to detect NTG in tablets and serum. Two kinds of immunogens were synthesized using ethylcarbodiimide (EDC)/hydroxysuccinimide (NHS) and carbonyldiimidazole (CDI)/4-dimethylaminopyridine (DMAP) as coupling reagents respectively. When activated by EDC/NHS, more molecules of NTG coupled with carrier protein in immunogens. A horseradish peroxidase (HRP)-luminol-H2O2 system with p-iodophenol enhancement was applied in the CLIA analysis. The antibodies in EDC/NHS group showed higher titer, sensitivity and wider detection linear range than those in CDI/DMAP group, and were chosen for next studies. The developed CLIA assay exhibited good selectivity towards NTG among structually similar analogs. The method could detect as low as 0.35 ng mL(-1) NTG in buffer, 2.1 ng mL(-1) NTG in serum and 0.84 ng mL(-1) NTG in tablets. The CLIA method provided consistent results with HPLC method (r=0.9986) in determination of NTG from 5.0 to 400 microg mL(-1). The CLIA method could detect 78 samples in one assay, and the samples need only dilution in pretreatment. As a summary, this research offers a sensitive assay for high-throughout screening of NTG in formulation control and pharmacokinetic studies.
Development and in vitro/in vivo evaluation of controlled release provesicles of a nateglinide-maltodextrin complex.[Pubmed:26579411]
Acta Pharm Sin B. 2014 Oct;4(5):408-16.
The aim of this study was to characterize the provesicle formulation of Nateglinide (NTG) to facilitate the development of a novel controlled release system of NTG with improved efficacy and oral bioavailability compared to the currently marketed NTG formulation (Glinate 60). NTG provesicles were prepared by a slurry method using the non-ionic surfactant, Span 60 (SP), and cholesterol (CH) as vesicle forming agents and maltodextrin as a coated carrier. Multilamellar niosomes with narrow size distribution were shown to be successfully prepared by means of dynamic laser scattering (DLS) and field emission scanning electron microscopy (FESEM). The absence of drug-excipient interactions was confirmed by Fourier transform infrared spectroscopy (FT-IR), differential scanning calorimetry (DSC) and X-ray diffraction (XRD) studies. In vitro release of NTG in different dissolution media was improved compared to pure drug. A goat intestinal permeation study revealed that the provesicular formulation (F4) with an SP:CH ratio of 5:5 gave higher cumulative amount of drug permeated at 48 h compared to Glinate 60 and control. A pharmacodynamic study in streptozotocin-induced diabetic rats confirmed that formulation F4 significantly (P<0.05) reduced blood glucose levels in comparison to Glinate 60. Overall the results show that controlled release NTG provesicles offer a useful and promising oral delivery system for the treatment of type II diabetes.
Nateglinide, a D-phenylalanine derivative lacking either a sulfonylurea or benzamido moiety, specifically inhibits pancreatic beta-cell-type K(ATP) channels.[Pubmed:12604678]
J Pharmacol Exp Ther. 2003 Mar;304(3):1025-32.
A novel antidiabetic agent, Nateglinide, is a D-phenylalanine derivative lacking either a sulfonylurea or benzamido moiety. We examined with the patch-clamp method the effect of Nateglinide on recombinant ATP-sensitive K(+) (K(ATP)) channels expressed in human embryonic kidney 293T cells transfected with a Kir6.2 subunit and either of a sulfonylurea receptor (SUR) 1, SUR2A, and SUR2B. In inside-out patches, Nateglinide reversibly inhibited the spontaneous openings of all three types of SUR/Kir6.2 channels. Nateglinide inhibited SUR1/Kir6.2 channels with high and low affinities (K(i) = 75 nM and 114 microM) but SUR2A/Kir6.2 and SUR2B/Kir6.2 channels only with low affinity (K(i) = 105 and 111 microM, respectively). Nateglinide inhibited the K(ATP) current mediated by Kir6.2 lacking C-terminal 26 amino acids only with low affinity (K(i) = 290 microM) in the absence of SUR. Replacement of serine at position 1237 of SUR1 to tyrosine [SUR1(S1237Y)] specifically abolished the high-affinity inhibition of SUR1/Kir6.2 channels by Nateglinide. MgADP or MgUDP (100 microM) augmented the inhibitory effect of Nateglinide on SUR1/Kir6.2 but not SUR1(S1237Y)/Kir6.2 or SUR2A/Kir6.2 channels. This augmenting effect of MgADP was also observed with the SUR1/Kir6.2(K185Q) channel, which was not inhibited by MgADP, but not with the SUR1(K1384A)/Kir6.2 channel, which was not activated by MgADP. These results indicate that therapeutic concentrations of Nateglinide (approximately 10 microM) may selectively inhibit pancreatic type SUR1/Kir6.2 channels through SUR1, especially when the channel is activated by intracellular MgADP, even though the agent does not contain either a sulfonylurea or benzamido moiety.
Tissue selectivity of antidiabetic agent nateglinide: study on cardiovascular and beta-cell K(ATP) channels.[Pubmed:10565863]
J Pharmacol Exp Ther. 1999 Dec;291(3):1372-9.
Nateglinide (NAT) stimulates insulin secretion from pancreatic beta-cells by closing K(ATP) channels. Because K(ATP) channels are widely distributed in cardiovascular (CV) tissues, we assessed the tissue specificity of NAT by examining its effect on K(ATP) channels in enzymatically isolated rat beta-cells, rat cardiac myocytes, and smooth muscle cells from porcine coronary artery and rat aorta with the patch-clamp method. The selectivity of known antidiabetic agents glyburide (GLY) and repaglinide (REP) was also studied for comparison. NAT was found to inhibit K(ATP) channels in the cells from porcine coronary artery and rat aorta with IC(50)s of 2.3 and 0. 3 mM, respectively, compared with 7.4 microM in rat beta-cells, indicating a respective 311- and 45-fold selectivity (p <.01) for beta-cells. With an IC(50) of 5.0 nM in beta-cells, REP displayed an approximately 16-fold (p <.05) selectivity for beta-cells over both types of vascular cells. GLY was nonselective between vascular and beta-cells. At equipotent concentrations (2x respective IC(50)s in beta-cells), NAT, GLY, and REP all caused 62% reduction of pancreatic K(ATP) current but a respective 39, 55, and 66% inhibition of cardiac K(ATP) current. These data collectively indicate that NAT, when compared with GLY and REP, at concentrations effective in stimulating insulin secretion is least likely to cause detrimental CV effects via blockade of CV K(ATP) channels.
The ability of a new hypoglycaemic agent, A-4166, compared to sulphonylureas, to increase cytosolic Ca2+ in pancreatic beta-cells under metabolic inhibition.[Pubmed:9105692]
Br J Pharmacol. 1997 Apr;120(7):1191-8.
1. N-(trans-4-isopropylcyclohexanecarbonyl)-D-phenylalanine (A-4166) is a new non-sulphonylurea oral hypoglycaemic agent which stimulates insulin release by increasing cytosolic Ca2+ concentration ([Ca2+]i) in beta-cells. 2. We studied comparative effects of A-4166 and sulphonylureas on [Ca2+]i, measured by dual-wavelength fura-2 microfluorometry, in single rat pancreatic beta-cells under normal conditions and conditions where glucose metabolism was inhibited. 3. A glucokinase inhibitor, mannoheptulose (10 mM), a mitochondrial respiratory inhibitor, KCN (100 microM), and uncouplers, dinitrophenol (DNP, 50 microM) and carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, 0.3 microM), were used to abolish glucose-induced increases in [Ca2+]i in a reversible manner. 4. Under control conditions, A-4166 was one order more potent than tolbutamide in increasing [Ca2+]i, and maximal responses were evoked by 30 microM A-4166 and 300 microM tolbutamide. These equipotent concentrations were employed for the comparative study where glucose metabolism was inhibited. 5. In the presence of mannoheptulose, [Ca2+]i responses to tolbutamide, but not those to A-4166, were attenuated in a reversible manner. 6. KCN, DNP and FCCP inhibited [Ca2+]i responses to tolbutamide to a much greater extent than those to A-4166. Responses to tolbutamide even at 3.3 times the equipotent concentration (1000 microM) were also markedly attenuated by these inhibitors. Responses evoked by another sulphonylurea, gliclazide, were inhibited by DNP to a larger extent than A-4166-induced responses. 7. The results indicate that A-4166 acts more effectively than sulphonylureas to increase [Ca2+]i in beta-cells during metabolic inhibition.
N-(cyclohexylcarbonyl)-D-phenylalanines and related compounds. A new class of oral hypoglycemic agents. 2.[Pubmed:2738878]
J Med Chem. 1989 Jul;32(7):1436-41.
A series of analogues of N-(cyclohexylcarbonyl)-D-phenylalanine (5) have been synthesized and evaluated for their hypoglycemic activity. Relationships were studied between the activity and the three-dimensional structure of the acyl moiety, which was characterized by high-resolution 1H NMR spectroscopy and MNDO calculations. The role of the carboxyl group of the phenylalanine moiety was also studied by comparing the activities of the enantiomers, the decarboxyl derivative, the esters, and the amides of the phenylalanine derivatives. Thus, the structural requirements for possessing hypoglycemic activity was elucidated and a highly active compound, N-[(trans-4-isopropylcyclohexyl)carbonyl]-D-phenylalanine (13) was obtained, which showed a 20% blood glucose decrease at an oral dose of 1.6 mg/kg in fasted normal mice.


