PJ34PARP-l inhibitor CAS# 344458-19-1 |

- PJ34 hydrochloride
Catalog No.:BCC2210
CAS No.:344458-15-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure
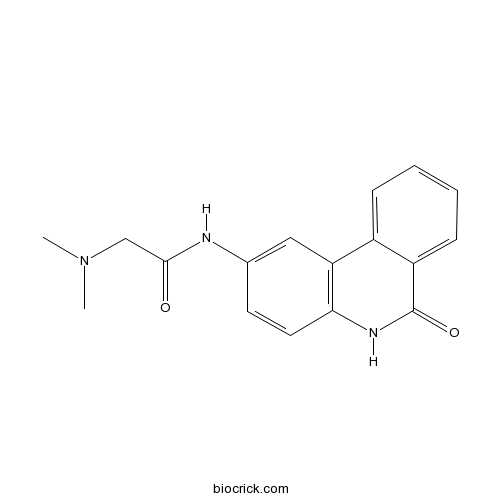
3D structure
| Cas No. | 344458-19-1 | SDF | Download SDF |
| PubChem ID | 4858 | Appearance | Powder |
| Formula | C17H17N3O2 | M.Wt | 295.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 30 mg/mL (101.58 mM; Need ultrasonic and warming) | ||
| Chemical Name | 2-(dimethylamino)-N-(6-oxo-5H-phenanthridin-2-yl)acetamide | ||
| SMILES | CN(C)CC(=O)NC1=CC2=C(C=C1)NC(=O)C3=CC=CC=C32 | ||
| Standard InChIKey | UYJZZVDLGDDTCL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H17N3O2/c1-20(2)10-16(21)18-11-7-8-15-14(9-11)12-5-3-4-6-13(12)17(22)19-15/h3-9H,10H2,1-2H3,(H,18,21)(H,19,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PJ34 is a novel potent specific inhibitor of PARP-l/2 with EC50 of 20 nM. | |||||
| Targets | PARP | |||||
| IC50 | 20 nM (EC50) | |||||

PJ34 Dilution Calculator

PJ34 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3859 mL | 16.9296 mL | 33.8593 mL | 67.7186 mL | 84.6482 mL |
| 5 mM | 0.6772 mL | 3.3859 mL | 6.7719 mL | 13.5437 mL | 16.9296 mL |
| 10 mM | 0.3386 mL | 1.693 mL | 3.3859 mL | 6.7719 mL | 8.4648 mL |
| 50 mM | 0.0677 mL | 0.3386 mL | 0.6772 mL | 1.3544 mL | 1.693 mL |
| 100 mM | 0.0339 mL | 0.1693 mL | 0.3386 mL | 0.6772 mL | 0.8465 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50 Value: 20 nM(EC50)[1] PJ34 (hydrochloride) is a novel potent specific inhibitor of PARP-l. PJ34 has been reported to enhance chemotherapeutic effects in certain types of tumors. in vitro: PJ34 inhibited peroxynitrite-induced cell necrosis with EC50 of 20 nM. PJ34 provides cardioprotection by decreasing myocardial infarct size and enhancing postischemic regional and global functional recovery [1]. Treatment with PJ34 increased NIS promoter activity without affecting PARP-1 binding to the promoter sequence, in addition to an increase of histone modification activation marks (H3K9K14ac, H3K4me3) [2]. in vivo: In a model of systemic endotoxemia, PJ34 pretreatment significantly reduced plasma levels of TNF-alpha, IL-1beta and nitrite/nitrate (breakdown products of nitric oxide) production. PJ34 treatment (oral gavage) induced a significant suppression of the inflammatory response in dextran sulfate colitis, multiple low dose streptozotocin diabetes [3]. The PJ34 showed significant reduction on infarct size (37.5%+/-4.5% and 50.5%+/-4.8% of the area at risk) for PJ34 and control pigs groups, respectively, (p < 0.05) [4]. Clinical trial: N/A
- PJ34 hydrochloride
Catalog No.:BCC2210
CAS No.:344458-15-7
- Nortrachelogenin
Catalog No.:BCN5280
CAS No.:34444-37-6
- Amycomycin
Catalog No.:BCN1824
CAS No.:344362-08-9
- Alpha-Onocerin diacetate
Catalog No.:BCN6700
CAS No.:34434-99-6
- Prudomestin
Catalog No.:BCN5279
CAS No.:3443-28-5
- Tirandamycin A
Catalog No.:BCN1861
CAS No.:34429-70-4
- Ligularine
Catalog No.:BCN2090
CAS No.:34429-54-4
- Ikshusterol
Catalog No.:BCN5278
CAS No.:34427-61-7
- Lyoniside
Catalog No.:BCN5277
CAS No.:34425-25-7
- 2,3-dihydrosciadopitysin
Catalog No.:BCN4034
CAS No.:34421-19-7
- Lathyrol
Catalog No.:BCN4963
CAS No.:34420-19-4
- 8-Chloroadenosine
Catalog No.:BCC7935
CAS No.:34408-14-5
- 17-ODYA
Catalog No.:BCC6717
CAS No.:34450-18-5
- Leukadherin 1
Catalog No.:BCC6332
CAS No.:344897-95-6
- Araloside X
Catalog No.:BCN2467
CAS No.:344911-90-6
- SSR 69071
Catalog No.:BCC2369
CAS No.:344930-95-6
- Pseudoephedrine Hydrochloride; Threo-Ephedrine Hydrochloride
Catalog No.:BCC8241
CAS No.:345-78-8
- Myricanol triacetate
Catalog No.:BCN5281
CAS No.:34509-52-9
- 1,3,6-Trihydroxy-2,5-dimethoxyxanthone
Catalog No.:BCN7216
CAS No.:345287-92-5
- Arnicolide C
Catalog No.:BCN7978
CAS No.:34532-67-7
- Arnicolide D
Catalog No.:BCN7975
CAS No.:34532-68-8
- Beta,beta-Dimethylacrylalkannin
Catalog No.:BCN2767
CAS No.:34539-65-6
- 6,7-Dehydroferruginol
Catalog No.:BCN3218
CAS No.:34539-84-9
- Madecassoside
Catalog No.:BCN1012
CAS No.:34540-22-2
Protective effects of PARP inhibitor, PJ34, is related to down-regulation of calpain and NF-kappaB in a mouse model of TBI.[Pubmed:27119554]
Brain Inj. 2016 Apr 27:1-11.
BACKGROUND: Poly(ADP-ribose) polymerase (PARP), calpain and nuclear factor-kappaB (NF-kappaB) are reported to participate in inflammatory reactions in pathological conditions and are involved in traumatic brain injury. The objective of this study was to investigate whether PARP participated in inflammation related to calpain and NF-kappaB in a mouse model of controlled cortical impact (CCI). MATERIALS AND METHODS: PJ34 (10 mg kg(-1)), a selective PARP inhibitor, was administered intraperitoneally 5 minutes and 8 hours after experimental CCI. A neurobehavioural evaluation and a histopathological analysis were then performed and the contusion volume, calpain activity and protein levels were measured in all animals. RESULTS: Treatment with PJ34 markedly reduced neurological deficits, decreased contusion volume and attenuated necrotic and apoptotic neuronal cell death 24 hours after CCI. The data showed that the cytosolic and nuclear fractions of calpain and NF-kappaB were up-regulated in the injured cortex and that these changes were reversed by PJ34. Moreover, PJ34 significantly enhanced the calpastatin and IkappaB levels and decreased the levels of inflammatory mediators. CONCLUSIONS: PARP inhibition by PJ34 suppresses the over-activation of calpain and the production of inflammatory factors that are caused by NF-kappaB activation and it improves neurological functioning, decreases the contusion volume and attenuates neuronal cell death in a mouse model of CCI.
PARP Inhibitor PJ34 Suppresses Osteogenic Differentiation in Mouse Mesenchymal Stem Cells by Modulating BMP-2 Signaling Pathway.[Pubmed:26492236]
Int J Mol Sci. 2015 Oct 19;16(10):24820-38.
Poly(ADP-ribosyl)ation is known to be involved in a variety of cellular processes, such as DNA repair, cell death, telomere regulation, genomic stability and cell differentiation by poly(ADP-ribose) polymerase (PARP). While PARP inhibitors are presently under clinical investigation for cancer therapy, little is known about their side effects. However, PARP involvement in mesenchymal stem cell (MSC) differentiation potentiates MSC-related side effects arising from PARP inhibition. In this study, effects of PARP inhibitors on MSCs were examined. MSCs demonstrated suppressed osteogenic differentiation after 1 microM PJ34 treatment without cytotoxicity, while differentiation of MSCs into chondrocytes or adipocytes was unaffected. PJ34 suppressed mRNA induction of osteogenic markers, such as Runx2, Osterix, Bone Morphogenetic Protein-2, Osteocalcin, bone sialoprotein, and Osteopontin, and protein levels of Bone Morphogenetic Protein-2, Osterix and Osteocalcin. PJ34 treatment also inhibited transcription factor regulators such as Smad1, Smad4, Smad5 and Smad8. Extracellular mineralized matrix formation was also diminished. These results strongly suggest that PARP inhibitors are capable of suppressing osteogenic differentiation and poly(ADP-ribosyl)ation may play a physiological role in this process through regulation of BMP-2 signaling. Therefore, PARP inhibition may potentially attenuate osteogenic metabolism, implicating cautious use of PARP inhibitors for cancer treatments and monitoring of patient bone metabolism levels.
Frataxin Deficiency Promotes Excess Microglial DNA Damage and Inflammation that Is Rescued by PJ34.[Pubmed:26954031]
PLoS One. 2016 Mar 8;11(3):e0151026.
An inherited deficiency in the frataxin protein causes neurodegeneration of the dorsal root ganglia and Friedreich's ataxia (FA). Frataxin deficiency leads to oxidative stress and inflammatory changes in cell and animal models; however, the cause of the inflammatory changes, and especially what causes brain microglial activation is unclear. Here we investigated: 1) the mechanism by which frataxin deficiency activates microglia, 2) whether a brain-localized inflammatory stimulus provokes a greater microglial response in FA animal models, and 3) whether an anti-inflammatory treatment improves their condition. Intracerebroventricular administration of LPS induced higher amounts of microglial activation in the FA mouse model vs controls. We also observed an increase in oxidative damage in the form of 8-oxoguanine (8-oxo-G) and the DNA repair proteins MUTYH and PARP-1 in cerebellar microglia of FA mutant mice. We hypothesized that frataxin deficiency increases DNA damage and DNA repair genes specifically in microglia, activating them. siRNA-mediated frataxin knockdown in microglial BV2 cells clearly elevated DNA damage and the expression of DNA repair genes MUTYH and PARP-1. Frataxin knockdown also induced a higher level of PARP-1 in MEF cells, and this was suppressed in MUTYH-/- knockout cells. Administration of the PARP-1 inhibitor PJ34 attenuated the microglial activation induced by intracerebroventricular injection of LPS. The combined administration of LPS and angiotensin II provoke an even stronger activation of microglia and neurobehavioral impairment. PJ34 treatment attenuated the neurobehavioral impairments in FA mice. These results suggest that the DNA repair proteins MUTYH and PARP-1 may form a pathway regulating microglial activation initiated by DNA damage, and inhibition of microglial PARP-1 induction could be an important therapeutic target in Friedreich's ataxia.
Synergistic suppressive effect of PARP-1 inhibitor PJ34 and HDAC inhibitor SAHA on proliferation of liver cancer cells.[Pubmed:26223923]
J Huazhong Univ Sci Technolog Med Sci. 2015 Aug;35(4):535-540.
Poly (ADP-ribose) polymerase-1 (PARP-1) inhibitors and histone deacetylase (HDAC) inhibitors have recently emerged as promising anticancer drugs. The aim of this study was to investigate the effect of combination treatment with the PARP inhibitor PJ34 and HDAC inhibitor SAHA on the proliferation of liver cancer cells. Cell proliferation and apoptosis were assessed in three human liver cancer cell lines (HepG2, Hep3B and HCC-LM3) treated with PJ34 (8 mumol/L) and SAHA (1 mumol/L), alone or combined, by Cell Counting Kit-8 assay and flow cytometry, respectively. The nude mice bearing subcutaneous HepG2 tumors were administered different groups of drugs (10 mg/kg PJ34, 25 mg/kg SAHA, 10 mg/kg PJ34+25 mg/kg SAHA), and the inhibition rates of tumor growth were compared between groups. The results showed that combined use of PJ34 and SAHA could synergistically inhibit the proliferation of liver cancer cell lines HepG2, Hep3B and HCC-LM3. The apoptosis rate of HepG2 cells treated with PJ34+SAHA was significantly higher than that of HepG2 cells treated with PJ34 or SAHA alone (P<0.05). In vivo, the tumor inhibition rates were 53.5%, 61.4% and 82.6% in PJ34, SAHA and PJ34+SAHA groups, respectively. The combined use of PJ34 and SAHA could significantly inhibit the xenograft tumor growth when compared with use of PJ34 or SAHA alone (P<0.05). It was led to conclude that PJ34 and SAHA can synergistically suppress the proliferation of liver cancer cells.


