PPTCAS# 263717-53-9 |
- BIBR 953 (Dabigatran, Pradaxa)
Catalog No.:BCC2139
CAS No.:211914-51-1
- BIBR-1048
Catalog No.:BCC3738
CAS No.:211915-06-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 263717-53-9 | SDF | Download SDF |
| PubChem ID | 6095481 | Appearance | Powder |
| Formula | C24H22N2O3 | M.Wt | 386.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (258.77 mM) *"≥" means soluble, but saturation unknown. | ||
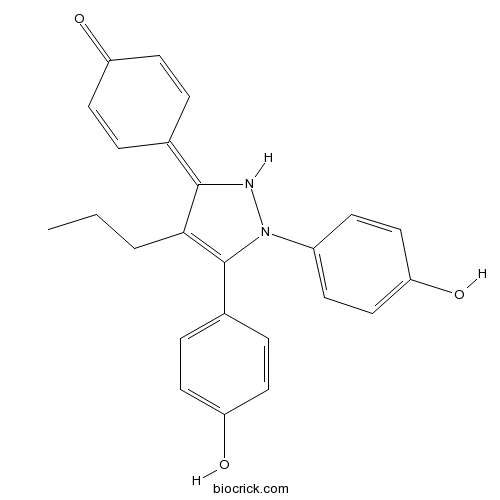
| Chemical Name | 4-[2,3-bis(4-hydroxyphenyl)-4-propyl-1H-pyrazol-5-ylidene]cyclohexa-2,5-dien-1-one | ||
| SMILES | CCCC1=C(N(NC1=C2C=CC(=O)C=C2)C3=CC=C(C=C3)O)C4=CC=C(C=C4)O | ||
| Standard InChIKey | UOSWGERPQQOSHS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H22N2O3/c1-2-3-22-23(16-4-10-19(27)11-5-16)25-26(18-8-14-21(29)15-9-18)24(22)17-6-12-20(28)13-7-17/h4-15,25,28-29H,2-3H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, subtype-selective estrogen receptor agonist (EC50 ~ 200 pM); displays 410-fold selectivity for ERα over ERβ. Prevents ovariectomy-induced weight gain and loss of bone mineral density, and induces gene expression in the hypothalamus following systemic administration in vivo. |

PPT Dilution Calculator

PPT Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5877 mL | 12.9383 mL | 25.8766 mL | 51.7531 mL | 64.6914 mL |
| 5 mM | 0.5175 mL | 2.5877 mL | 5.1753 mL | 10.3506 mL | 12.9383 mL |
| 10 mM | 0.2588 mL | 1.2938 mL | 2.5877 mL | 5.1753 mL | 6.4691 mL |
| 50 mM | 0.0518 mL | 0.2588 mL | 0.5175 mL | 1.0351 mL | 1.2938 mL |
| 100 mM | 0.0259 mL | 0.1294 mL | 0.2588 mL | 0.5175 mL | 0.6469 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ESI-09
Catalog No.:BCC5504
CAS No.:263707-16-0
- Stachysterone D
Catalog No.:BCC8362
CAS No.:26361-67-1
- H-Lys-OMe .2HCl
Catalog No.:BCC2981
CAS No.:26348-70-9
- Rhodojaponin III
Catalog No.:BCN2809
CAS No.:26342-66-5
- H-Arg-OMe.2HCl
Catalog No.:BCC2861
CAS No.:26340-89-6
- 1,2-Benzisothiazolin-3-one
Catalog No.:BCC8412
CAS No.:2634-33-5
- Aesculuside B
Catalog No.:BCC8115
CAS No.:26339-92-4
- Escin IB
Catalog No.:BCN2970
CAS No.:26339-90-2
- 4-(cis)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1468
CAS No.:263368-92-9
- 4-(trans)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1469
CAS No.:263368-91-8
- (S)-(-)-Pindolol
Catalog No.:BCC6916
CAS No.:26328-11-0
- Perilloxin
Catalog No.:BCN6614
CAS No.:263249-77-0
- EMDT oxalate
Catalog No.:BCC7888
CAS No.:263744-72-5
- Buergerinin G
Catalog No.:BCN4659
CAS No.:263764-83-6
- 3,27-Dihydroxy-20(29)-lupen-28-oic acid methyl ester
Catalog No.:BCN1467
CAS No.:263844-79-7
- 3-Acetoxy-27-hydroxy-20(29)-lupen-28-oic acid methyl ester
Catalog No.:BCN1466
CAS No.:263844-80-0
- AG 045572
Catalog No.:BCC7464
CAS No.:263847-55-8
- S 25585
Catalog No.:BCC7687
CAS No.:263849-50-9
- DPPA (Kg)
Catalog No.:BCC2690
CAS No.:26386-88-9
- Z-Orn-OH
Catalog No.:BCC2757
CAS No.:2640-58-6
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- Methyl 5-{2-[(tert-butylamino)carbothioyl]carbohydrazonoyl}-1-(2,4-difluorophenyl)-1H-pyrazole-4-carboxylate
Catalog No.:BCC7906
CAS No.:264233-05-8
- 6',7'-Dihydroxybergamottin
Catalog No.:BCN5142
CAS No.:264234-05-1
- Oxypeucedanin hydrate
Catalog No.:BCN2698
CAS No.:2643-85-8
Determination of cadmium and lead at sub-ppt level in soft drinks: An efficient combination between dispersive liquid-liquid microextraction and graphite furnace atomic absorption spectrometry.[Pubmed:27979292]
Food Chem. 2017 Apr 15;221:907-912.
A DLLME method for extraction and preconcentration of Cd and Pb from soft drinks and further determination by GF AAS was developed. Important parameters of DLLME such as the type and volume of dispersive and extraction solvents, concentration of DDTC (complexing agent) and pH were evaluated. Better results were obtained using 500muL of acetone for Cd and 700muL of acetonitrile for Pb as dispersive solvents, 60muL of CCl4 as extraction solvent for both analytes and 500muL of 1.5% DDTC solution. Accuracy was evaluated by recovery assays and ranged from 91 to 113% for Cd and from 95 to 108% for Pb, with RSD below 10 and 7%, respectively. The LODs were 0.006 and 0.072ngL(-1) for Cd and Pb, respectively. The optimized method was applied for the determination of Cd and Pb in soft drinks with different brands and flavours.
Pesticide analysis at ppt concentration levels: coupling nano-liquid chromatography with dielectric barrier discharge ionization-mass spectrometry.[Pubmed:26898206]
Anal Bioanal Chem. 2016 May;408(13):3425-34.
We report the coupling of nano-liquid chromatography (nano-LC) with an ambient dielectric barrier discharge ionization (DBDI)-based source. Detection and quantification were carried out by high-resolution mass spectrometry (MS), using an LTQ-Orbitrap in full scan mode. Despite the fact that nano-LC systems are rarely used in food analysis, this coupling was demonstrated to deliver extremely high sensitivity in pesticide analysis, with limits of detection (LODs) as low as 10 pg/mL. In all cases, the limits of quantification (LOQs) were compliant with the current EU regulation. An excellent signal linearity over up to four orders of magnitude was also observed. Therefore, this method can easily compete with conventional GC-(EI)-MS or LC-ESI-MS/MS methods and in some cases outperform them. The method was successfully tested for food sample analysis, with apples and baby food, extracted using the QuEChERS approach. Our results demonstrate an outstanding sensitivity (at femtogram level) and reproducibility of the nano-LC-DBDI coupling, capable of improving routine pesticide analysis. To the best of our knowledge, this is the most sensitive and reproducible plasma-MS-based method for pesticide analysis reported to date.
Impact of utilisation of uncompleted handouts on power point presentations (PPT) in rural Indian medical institute.[Pubmed:27382583]
J Adv Med Educ Prof. 2016 Jul;4(3):145-9.
INTRODUCTION: Note taking while attending a PPT requires high activity of memory and writing process which ultimately leads to what is called "death by power point" referring to boredom and fatigue. To overcome this we planned to evaluate the impact of utilisation of uncompleted handouts given prior to PPT presentations. METHODS: Final year MBBS students were divided in 2 batches, batch A and batch B. For a set of lectures one batch was provided with handouts before lecture while the other batch was given lectures only. Crossover was done to avoid bias, all the lectures being given by the same presenter. At the end of each lecture, a short questionnaire of 10 Multiple Choice Question (MCQ) was provided to the students. Mean scores were calculated for lectures with handouts and without handouts. RESULTS: For a set of lectures, when batch A was provided with handouts, the mean score was 28.2; for batch B to which no handouts were given the mean score was 23.4. Similarly, for batch B when provided with handouts the mean score was 29.1, for batch A which was not provided with handouts the mean score was 24. There was an average increase of 4.2 marks. Actual gain when handouts were provided was 1.2 marks per lecture. It was more for the batch comprising of repeater students as compared to the batch of fresher students. Increase in attendance was also noted. CONCLUSION: Providing uncompleted handouts before a didactic lecture definitely results in increase in knowledge gain; repeater students benefit more with uncompleted handouts.
Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand.[Pubmed:12399409]
Endocrinology. 2002 Nov;143(11):4172-7.
Estrogens elicit many biomedically important responses in different target tissues, and the respective roles of the two estrogen receptors, ERalpha and ERbeta, in mediating these bioactivities is incompletely understood. In this study, we investigated the activity of an ERalpha-selective agonist ligand, propyl pyrazole triol (PPT), in several rat animal models to define the involvement of ERalpha in these biological responses. In a short-term (4 d) uterotrophic assay, PPT was found to be as efficacious as 17alpha-ethinyl-17beta-estradiol in stimulating uterine weight gain and up-regulating complement 3 gene expression. In a 6-wk chronic model, PPT completely prevented the ovariectomy-induced body weight increase and loss of bone mineral density. It also increased uterine weight and markedly reduced plasma cholesterol levels in these mature animals. PPT was also effective in the brain. It increased progesterone receptor mRNA in the arcuate and ventromedial nuclei of the hypothalamus and prevented experimentally induced hot flushes. Our findings indicate that several physiologically relevant estrogen-induced tissue responses can be effectively evoked via ERalpha alone. By providing an approach that is complementary to that of analyzing the phenotype and response of ER knockout animals, our findings also demonstrate that ER subtype-selective ligands can play a valuable role in enhancing our understanding of how estrogens work through the two ER subtypes.
Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-alpha and estrogen receptor-beta: correlations with biological character and distinct differences among SRC coactivator family members.[Pubmed:11014206]
Endocrinology. 2000 Oct;141(10):3534-45.
Ligands for the estrogen receptor (ER) that have the capacity to selectively bind to or activate the ER subtypes ERalpha or ERbeta would be useful in elucidating the biology of these two receptors and might assist in the development of estrogen pharmaceuticals with improved tissue selectivity. In this study, we examine three compounds of novel structure that act as ER subtype-selective ligands. These are a propyl pyrazole triol (PPT), which is a potent agonist on ERalpha but is inactive on ERbeta, and a pair of substituted tetrahydrochrysenes (THC), one enantiomer of which (S,S-THC) is an agonist on both ERalpha and ERbeta, the other (R,R-THC) being an agonist on ERalpha but an antagonist on ERbeta. To investigate the molecular mechanisms underlying the ER subtype-selective actions of these compounds, we have determined the conformational changes induced in ERalpha and ERbeta by these ligands using protease digestion sensitivity, and we have tested the ability of these ligands to promote the recruitment of representatives of the three SRC/p160 coactivator protein family members (SRC-1, GRIP-1, ACTR, respectively) to ERalpha and ERbeta using yeast two-hybrid and glutathione-S-transferase (GST) pull-down assays. We find that the ligand-ER protease digestion pattern is distinctly different for stimulatory and inhibitory ligands, and that this assay, as well as coactivator recruitment, are excellent indicators of their agonist/antagonist character. Interestingly however, compared with estradiol, the novel agonist ligands show some quantitative differences in their ability to recruit SRC-1, -2, and -3. This implies that while generally similar to estradiol, these ligands induce ER conformations that differ somewhat from that induced by estradiol, differences that are illustrative of the nature of their biological character. The application of methods to characterize the conformations induced in ER subtypes by novel ligands, as done in this study, enables a greater understanding of how ligand-receptor conformations relate to estrogen agonist or antagonist behavior.
Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists.[Pubmed:11150164]
J Med Chem. 2000 Dec 28;43(26):4934-47.
We have found that certain tetrasubstituted pyrazoles are high-affinity ligands for the estrogen receptor (ER) (Fink et al. Chem. Biol. 1999, 6, 205-219) and that one pyrazole is considerably more potent as an agonist on the ERalpha than on the ERbeta subtype (Sun et al. Endocrinology 1999, 140, 800-804). To investigate what substituent pattern provides optimal ER binding affinity and the greatest enhancement of potency as an ERalpha-selective agonist, we prepared a number of tetrasubstituted pyrazole analogues with defined variations at certain substituent positions. Analysis of their binding affinity pattern shows that a C(4)-propyl substituent is optimal and that a p-hydroxyl group on the N(1)-phenyl group also enhances affinity and selectivity for ERalpha. The best compound in this series, a propylpyrazole triol (PPT, compound 4g), binds to ERalpha with high affinity (ca. 50% that of estradiol), and it has a 410-fold binding affinity preference for ERalpha. It also activates gene transcription only through ERalpha. Thus, this compound represents the first ERalpha-specific agonist. We investigated the molecular basis for the exceptional ERalpha binding affinity and potency selectivity of pyrazole 4g by a further study of structure-affinity relationships in this series and by molecular modeling. These investigations suggest that the pyrazole triols prefer to bind to ERalpha with their C(3)-phenol in the estradiol A-ring binding pocket and that binding selectivity results from differences in the interaction of the pyrazole core and C(4)-propyl group with portions of the receptor where ERalpha has a smaller residue than ERbeta. These ER subtype-specific interactions and the ER subtype-selective ligands that can be derived from them should prove useful in defining those biological activities in estrogen target cells that can be selectively activated through ERalpha.


