Pomalidomide (CC-4047)Immunomodulator,antumor/anti-angiogenic CAS# 19171-19-8 |
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
Quality Control & MSDS
Number of papers citing our products

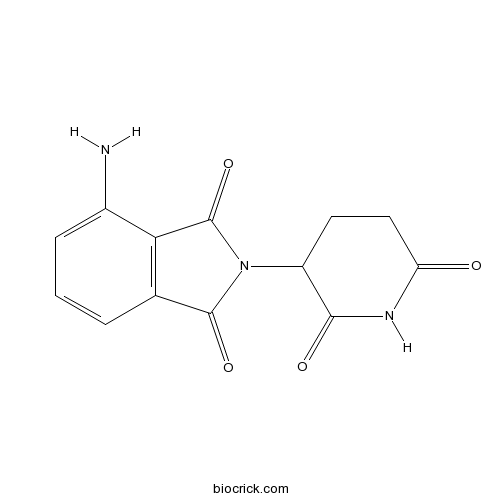
Chemical structure
3D structure
| Cas No. | 19171-19-8 | SDF | Download SDF |
| PubChem ID | 134780 | Appearance | Powder |
| Formula | C13H11N3O4 | M.Wt | 273.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CC-4047 | ||
| Solubility | DMSO : ≥ 100 mg/mL (365.98 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-amino-2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione | ||
| SMILES | C1CC(=O)NC(=O)C1N2C(=O)C3=C(C2=O)C(=CC=C3)N | ||
| Standard InChIKey | UVSMNLNDYGZFPF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H11N3O4/c14-7-3-1-2-6-10(7)13(20)16(12(6)19)8-4-5-9(17)15-11(8)18/h1-3,8H,4-5,14H2,(H,15,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pomalidomide is an inhibitor of LPS-induced TNF-α release with IC50 of 13 nM. | |||||
| Targets | TNF-α | |||||
| IC50 | 13 nM | |||||

Pomalidomide (CC-4047) Dilution Calculator

Pomalidomide (CC-4047) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6603 mL | 18.3016 mL | 36.6032 mL | 73.2064 mL | 91.5081 mL |
| 5 mM | 0.7321 mL | 3.6603 mL | 7.3206 mL | 14.6413 mL | 18.3016 mL |
| 10 mM | 0.366 mL | 1.8302 mL | 3.6603 mL | 7.3206 mL | 9.1508 mL |
| 50 mM | 0.0732 mL | 0.366 mL | 0.7321 mL | 1.4641 mL | 1.8302 mL |
| 100 mM | 0.0366 mL | 0.183 mL | 0.366 mL | 0.7321 mL | 0.9151 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pomalidomide, previously known as CC-4047 or actimid, is a potent immunomodulatory molecule that exhibits antineoplastic activity for the treatment of hematological malignancies, especially relapsed and refractory multiple myeloma (MM). As a derivative of thalidomide, pomalidomide has a similar chemical structure as thalidomide except for the addition of two oxo groups in the phthaloyl ring and an amino group at the fourth position. Generally, as an immunomodulatory molecule, pomalidomide demonstrates antitumor activity through a mechanism of blocking the tumor microenvironment by modulation of tumor-supporting cytokines (TNF-α, IL-6, IL-8 and VEGF), directly down-regulating key functions of tumor cells, and engaging support from non-immune host cells.
Reference
AA Chanan-Khan, A Swaika, A Paulus, SK Kumar, JR Mikhael, SV Rajkumar, A Dispenzieri, and MQ Lacy. Pomalidomide: the new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer Journal (2013) 3, el43
Evangelos Terpos, Nikolaos Kanellias, Dimitrios Christoulas, Efstathios Kastritis, and Meletios A Dimopoulos. Pomalidomide: a novel drug to treat relapsed and refractory multiple myeloma. Onco Targets Ther 2013; 6: 531-538
- SIB 1553A hydrochloride
Catalog No.:BCC6284
CAS No.:191611-89-9
- Trimethylgallic acid methyl ester
Catalog No.:BCN3369
CAS No.:1916-07-0
- 2-Deacetoxytaxinine B
Catalog No.:BCN1181
CAS No.:191547-12-3
- Epieriocalyxin A
Catalog No.:BCN1180
CAS No.:191545-24-1
- 2-Hydroxyxanthone
Catalog No.:BCN7545
CAS No.:1915-98-6
- Isoficusin A
Catalog No.:BCN6865
CAS No.:1914963-20-4
- LY 379268
Catalog No.:BCC7368
CAS No.:191471-52-0
- AGN 195183
Catalog No.:BCC5419
CAS No.:191469-29-1
- 3-Benzoylpropionic acid
Catalog No.:BCN1928
CAS No.:2051-95-8
- Fmoc-Tyr(HPO3Bzl)-OH
Catalog No.:BCC3565
CAS No.:191348-16-0
- Ursolic aldehyde
Catalog No.:BCN7712
CAS No.:19132-81-1
- 6-Deoxy-3-O-methyl-beta-allopyranosyl(1-4)-beta-cymaronic acid delta-lactone
Catalog No.:BCN1514
CAS No.:19131-13-6
- Tenacissoside G
Catalog No.:BCN4682
CAS No.:191729-43-8
- Tenacissoside I
Catalog No.:BCN4681
CAS No.:191729-44-9
- Tenacissoside H
Catalog No.:BCN2570
CAS No.:191729-45-0
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Cyclo(Pro-Gly)
Catalog No.:BCN2417
CAS No.:19179-12-5
- Deoxypodophyllotoxin
Catalog No.:BCN1182
CAS No.:19186-35-7
- BIBO 3304 trifluoroacetate
Catalog No.:BCC7355
CAS No.:191868-14-1
- Flutax 1
Catalog No.:BCC7298
CAS No.:191930-58-2
- NTNCB hydrochloride
Catalog No.:BCC7270
CAS No.:191931-56-3
- HIV-1 Tat Protein Peptide
Catalog No.:BCC4417
CAS No.:191936-91-1
- 12-Hydroxymyricanone
Catalog No.:BCN8046
CAS No.:191999-68-5
- Hinokiflavone
Catalog No.:BCN2989
CAS No.:19202-36-9
A phase I, dose-escalation study of pomalidomide (CC-4047) in combination with gemcitabine in metastatic pancreas cancer.[Pubmed:21051221]
Eur J Cancer. 2011 Jan;47(2):199-205.
INTRODUCTION: Pomalidomide is an investigational immunomodulating drug (IMiD) that also inhibits angiogenesis and has direct anti-tumour effects. This phase I study was performed to identify the optimal dose of pomalidomide to be used in combination with gemcitabine in the treatment of patients with metastatic pancreatic cancer. METHODS: Eligible patients had histologically documented metastatic adenocarcinoma of the pancreas. No prior gemcitabine for metastatic disease or for primary treatment of locally advanced disease was allowed although prior radiation therapy with 5-flourouracil (5-FU) or gemcitabine as a radiosensitizer was allowed. All patients received gemcitabine 1000 mg/m(2) IV on days 1, 8 and 15 of a 28 day cycle. Pomalidomide was administered orally on days 1-21 at doses escalated from 2 to 10mg daily. Patients were re-evaluated every 8 weeks; treatment continued until disease progression or intolerable toxicity occurred. RESULTS: Twenty-three patients were enrolled with a median age of 62 and Eastern Cooperative Oncology Group (ECOG) performance status 0 (87%) and 1 (13%). The maximum tolerated dose (MTD) was 10mg/day on days 1-21. Neutropaenia was the most common grade 3/4 toxicity (38%); other grade 3/4 toxicity included deep vein thrombosis (DVT) (22%) and anaemia (9%). While efficacy was not a primary end-point of this study, 3 of 20 evaluable patients (15%) had partial responses and 10 patients (50%) had >50% decrease in CA 19-9 levels. CONCLUSIONS: The combination of pomalidomide and gemcitabine was feasible and safe in most patients receiving first-line chemotherapy for metastatic pancreatic cancer. Neutropaenia, the dose-limiting toxicity, was brief and reversible. Intermittent dosing of pomalidomide allowed substantially higher doses than were previously reported with a continuous schedule. This combination merits further evaluation in the treatment of metastatic pancreatic cancer.
A phase I study of pomalidomide (CC-4047) in combination with cisplatin and etoposide in patients with extensive-stage small-cell lung cancer.[Pubmed:23370364]
J Thorac Oncol. 2013 Apr;8(4):423-8.
INTRODUCTION: This phase I/IIA study evaluated the maximum-tolerated dose (MTD), safety, and clinical benefit of pomalidomide, an immunomodulatory drug (IMiD), combined with cisplatin+etoposide chemotherapy, in treatment-naive patients with extensive-stage (ES) small-cell lung cancer (SCLC). METHODS: In this multicenter, open-label, dose-escalation study, patients received 21-day cycles of oral pomalidomide (1, 3, 5, and 4 mg/day) on days 1 to 14, plus cisplatin 25 mg/m and etoposide 100 mg/m administered intravenously on days 1 to 3; the MTD was determined during cycle 1 (standard 3+3 dose-escalation design), followed by a five-cycle extension phase. RESULTS: Twenty-two patients with ES SCLC, with a median age of 64.5 years received one or more doses of the study medication. Dose-limiting toxicities included grade 4 cerebral ischemia and grade 5 sepsis (1-mg cohort), grade 4 transient ischemic attack (5-mg cohort), and grade 5 neutropenic infection (5-mg cohort). The MTD for pomalidomide was 4 mg/day. In the MTD phase, the most common pomalidomide-related adverse events (AEs) were fatigue (72.7%), nausea (45.5%), and neutropenia (40.9%); 31.8% of patients experienced pomalidomide-related serious AEs and 40.9% cisplatin/etoposide-related serious AEs. Overall response rate was 31.8% (7 of 22); these were partial responses. Stable disease and progressive disease occurred in four patients (18.2%) each. The median response duration was 12.4 weeks. Median overall survival was 49.6 weeks. CONCLUSIONS: Pomalidomide at the MTD of 4 mg/day plus standard cisplatin+etoposide seems safe in treatment-naive patients with ES SCLC. However, addition of pomalidomide does not seem to improve the therapeutic index of chemotherapy alone.


