PyroxamideHDAC1 inhibitor CAS# 382180-17-8 |
- Pemetrexed
Catalog No.:BCC9115
CAS No.:137281-23-3
- Pralatrexate
Catalog No.:BCC2304
CAS No.:146464-95-1
- Pemetrexed disodium hemipenta hydrate
Catalog No.:BCC1844
CAS No.:357166-30-4
- Methotrexate
Catalog No.:BCC2301
CAS No.:59-05-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 382180-17-8 | SDF | Download SDF |
| PubChem ID | 4996 | Appearance | Powder |
| Formula | C13H19N3O3 | M.Wt | 265.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | N'-hydroxy-N-pyridin-3-yloctanediamide | ||
| SMILES | C1=CC(=CN=C1)NC(=O)CCCCCCC(=O)NO | ||
| Standard InChIKey | PTJGLFIIZFVFJV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H19N3O3/c17-12(15-11-6-5-9-14-10-11)7-3-1-2-4-8-13(18)16-19/h5-6,9-10,19H,1-4,7-8H2,(H,15,17)(H,16,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of histone deacetylase (HDAC); potently inhibits affinity purified HDAC1. Also inhibits the growth of tumor cells in vitro and in vivo. Induces p21/WAF1 expression in tumor cells. |

Pyroxamide Dilution Calculator

Pyroxamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7692 mL | 18.8459 mL | 37.6918 mL | 75.3835 mL | 94.2294 mL |
| 5 mM | 0.7538 mL | 3.7692 mL | 7.5384 mL | 15.0767 mL | 18.8459 mL |
| 10 mM | 0.3769 mL | 1.8846 mL | 3.7692 mL | 7.5384 mL | 9.4229 mL |
| 50 mM | 0.0754 mL | 0.3769 mL | 0.7538 mL | 1.5077 mL | 1.8846 mL |
| 100 mM | 0.0377 mL | 0.1885 mL | 0.3769 mL | 0.7538 mL | 0.9423 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
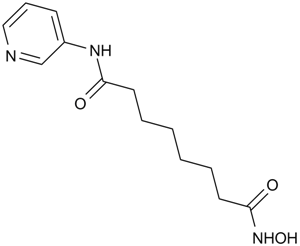
Pyroxamide is an inhibitor of histone deacetylase [1].
Histone deacetylases (HADC) are a series of enzymes that remove acetyl groups from an ε-N-acetyl lysine amino acid on a histone and make the histones to wrap the DNA more tightly, which prevent transcription.
In murine erythroleukemia (MEL) cells, pyroxamide induced terminal differentiation and inhibited cells growth by apoptosis or cell cycle arrest. Also, pyroxamide increased the levels of acetylated histones H2A, H2B, H3 and H4. Pyroxamide inhibited HDAC1 activity with ID50 value of 100 nM [1]. In RD (embryonal) and RH30B cell lines, pyroxamide (1.25-20.0 μM) induced apoptosis and accumulation of acetylated histones. Also, pyroxamide induced the expression of p21/WAF1 protein and increased the sub-G1 fraction [2].
In mice bearing human CWR22 prostate cancer xenograft, pyroxamide (100 or 200 mg/kg) significantly suppressed the growth of the tumor and increased the expression of p21/WAF1 protein in a dose-dependent way [1].
References:
[1]. Butler LM, Webb Y, Agus DB, et al. Inhibition of transformed cell growth and induction of cellular differentiation by pyroxamide, an inhibitor of histone deacetylase. Clin Cancer Res, 2001, 7(4): 962-970.
[2]. Kutko MC, Glick RD, Butler LM, et al. Histone deacetylase inhibitors induce growth suppression and cell death in human rhabdomyosarcoma in vitro. Clin Cancer Res, 2003, 9(15): 5749-5755.
- Coumarin VI
Catalog No.:BCN7833
CAS No.:38215-36-0
- Bacopaside II
Catalog No.:BCC8125
CAS No.:382146-66-9
- Adrenosterone
Catalog No.:BCC4061
CAS No.:382-45-6
- Sulindac
Catalog No.:BCC4861
CAS No.:38194-50-2
- 7,8-Dihydroxyflavone
Catalog No.:BCC6072
CAS No.:38183-03-8
- Bephenium Hydroxynaphthoate
Catalog No.:BCC3735
CAS No.:3818-50-6
- 28-Hydroxy-3-oxoolean-12-en-29-oic acid
Catalog No.:BCN1449
CAS No.:381691-22-1
- Brevianamide F
Catalog No.:BCN6452
CAS No.:38136-70-8
- Streptomycin sulfate
Catalog No.:BCC4851
CAS No.:3810-74-0
- Perampanel
Catalog No.:BCC1847
CAS No.:380917-97-5
- TACA
Catalog No.:BCC6564
CAS No.:38090-53-8
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- Filixic acid ABA
Catalog No.:BCN6330
CAS No.:38226-84-5
- Anhydroicaritin
Catalog No.:BCN5351
CAS No.:38226-86-7
- Enhydrin chlorohydrin
Catalog No.:BCN4639
CAS No.:38230-99-8
- beta-Amyrenonol
Catalog No.:BCN5436
CAS No.:38242-02-3
- 20(R)-Ginsenoside Rg3
Catalog No.:BCN5018
CAS No.:38243-03-7
- Malonomicin
Catalog No.:BCN1844
CAS No.:38249-71-7
- Glucose-conjugated MGMT inhibitor
Catalog No.:BCC1597
CAS No.:382607-78-5
- Burchellin
Catalog No.:BCN6676
CAS No.:38276-59-4
- Naringenin trimethyl ether
Catalog No.:BCN5437
CAS No.:38302-15-7
- Minoxidil
Catalog No.:BCC4297
CAS No.:38304-91-5
- YE 120
Catalog No.:BCC6188
CAS No.:383124-82-1
- 3-O-beta-D-Glucopyranosylplatycodigenin
Catalog No.:BCN7832
CAS No.:38337-25-6
Inhibition of transformed cell growth and induction of cellular differentiation by pyroxamide, an inhibitor of histone deacetylase.[Pubmed:11309347]
Clin Cancer Res. 2001 Apr;7(4):962-70.
PURPOSE: We have synthesized a series of hybrid polar compounds that induce differentiation and/or apoptosis of various transformed cells. These agents are also potent inhibitors of histone deacetylases (HDACs). Pyroxamide (suberoyl-3-aminopyridineamide hydroxamic acid) is a new member of this class of compounds that is currently under development as an anticancer agent. We investigated the activity of Pyroxamide as an inducer of differentiation and/or apoptosis in transformed cells. EXPERIMENTAL DESIGN AND RESULTS: Pyroxamide, at micromolar concentrations, induced terminal differentiation in murine erythroleukemia (MEL) cells and caused growth inhibition by cell cycle arrest and/or apoptosis in MEL, prostate carcinoma, bladder carcinoma, and neuroblastoma cells. Administration of Pyroxamide (100 or 200 mg/kg/day) to nude mice at doses that caused little evident toxicity significantly suppressed the growth of s.c. CWR22 prostate cancer xenografts. Despite the potent growth-inhibitory effects of Pyroxamide in this tumor model, serum prostate-specific antigen levels in control versus Pyroxamide-treated mice were not significantly different. Pyroxamide is a potent inhibitor of affinity-purified HDAC1 (ID(50) = 100 nM) and causes the accumulation of acetylated core histones in MEL cells cultured with the agent. Human CWR22 prostate tumor xenografts from mice treated with Pyroxamide (100 or 200 mg/kg/day) showed increased levels of histone acetylation and increased expression of the cell cycle regulator p21/WAF1, compared with tumors from vehicle-treated control animals. CONCLUSIONS: The findings suggest that Pyroxamide may be a useful agent for the treatment of malignancy and that induction of p21/WAF1 in transformed cells by Pyroxamide may contribute to the antitumor effects of this agent.
Chemical phylogenetics of histone deacetylases.[Pubmed:20139990]
Nat Chem Biol. 2010 Mar;6(3):238-243.
The broad study of histone deacetylases in chemistry, biology and medicine relies on tool compounds to derive mechanistic insights. A phylogenetic analysis of class I and II histone deacetylases (HDACs) as targets of a comprehensive, structurally diverse panel of inhibitors revealed unexpected isoform selectivity even among compounds widely perceived as nonselective. The synthesis and study of a focused library of cinnamic hydroxamates allowed the identification of, to our knowledge, the first nonselective HDAC inhibitor. These data will guide a more informed use of HDAC inhibitors as chemical probes and therapeutic agents.
Inhibitors of human histone deacetylase: synthesis and enzyme and cellular activity of straight chain hydroxamates.[Pubmed:11831887]
J Med Chem. 2002 Feb 14;45(4):753-7.
Inhibitors of histone deacetylase (HDAC) have been shown to induce terminal differentiation of human tumor cell lines and to have antitumor effects in vivo. We have prepared analogues of suberoylanilide hydroxamic acid (SAHA) and trichostatin A and have evaluated them in a human HDAC enzyme inhibition assay, a p21(waf1) (p21) promoter assay, and in monolayer growth inhibition assays. One compound, 4-(dimethylamino)-N-[7-(hydroxyamino)-7-oxoheptyl]-benzamide, was found to affect the growth of a panel of eight human tumor cell lines differentially.


