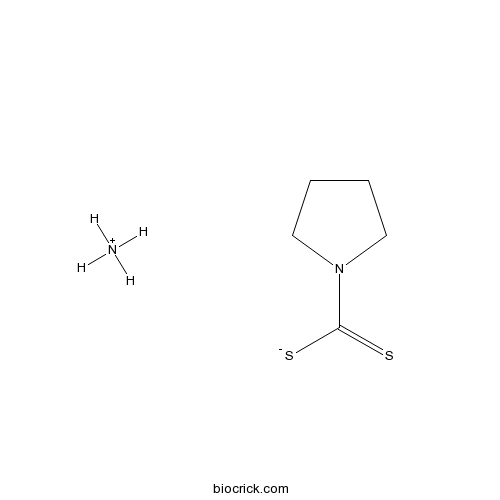
Pyrrolidinedithiocarbamate ammoniumCAS# 5108-96-3 |
- Pemetrexed
Catalog No.:BCC9115
CAS No.:137281-23-3
- Pralatrexate
Catalog No.:BCC2304
CAS No.:146464-95-1
- Pemetrexed disodium hemipenta hydrate
Catalog No.:BCC1844
CAS No.:357166-30-4
- Methotrexate
Catalog No.:BCC2301
CAS No.:59-05-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 5108-96-3 | SDF | Download SDF |
| PubChem ID | 517348 | Appearance | Powder |
| Formula | C5H12N2S2 | M.Wt | 164.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PDTC | ||
| Solubility | DMSO : ≥ 42 mg/mL (255.65 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | azanium;pyrrolidine-1-carbodithioate | ||
| SMILES | N.SC(=S)N1CCCC1 | ||
| Standard InChIKey | MDDIUTVUBYEEEM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H9NS2.H3N/c7-5(8)6-3-1-2-4-6;/h1-4H2,(H,7,8);1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | This inhibitor of nuclear factor κB (NF-κB) prevents the increase in NO-synthase mRNA by interleukin-1, without affecting the formation of NO-synthase mRNA produced by cAMP. |

Pyrrolidinedithiocarbamate ammonium Dilution Calculator

Pyrrolidinedithiocarbamate ammonium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0872 mL | 30.4358 mL | 60.8717 mL | 121.7434 mL | 152.1792 mL |
| 5 mM | 1.2174 mL | 6.0872 mL | 12.1743 mL | 24.3487 mL | 30.4358 mL |
| 10 mM | 0.6087 mL | 3.0436 mL | 6.0872 mL | 12.1743 mL | 15.2179 mL |
| 50 mM | 0.1217 mL | 0.6087 mL | 1.2174 mL | 2.4349 mL | 3.0436 mL |
| 100 mM | 0.0609 mL | 0.3044 mL | 0.6087 mL | 1.2174 mL | 1.5218 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pyrrolidinedithiocarbamate ammonium is a selective NF-κB inhibitor.
In Vitro:Pretreatment of cells with pyrrolidinedithiocarbamate (3-1000 μM) dose-dependently attenuate IL-8 production. Furthermore, pyrrolidinedithiocarbamate (100 μM) suppresses the accumulation of IL-8 mRNA. Pyrrolidinedithiocarbamate inhibits the activation of NF-κB, because pyrrolidinedithiocarbamate suppresses both NF-κB DNA binding and NF-κB-dependent transcriptional activity. NF-κB inhibition with pyrrolidinedithiocarbamate decrease IL-8 production by intestinal epithelial cells[1].
In Vivo:The DSS+pyrrolidinedithiocarbamate ammonium-treated groupII exhibits suppression of shortening of intestinal length and reduction of DAI score. Activated NF-κB level and IL-1β and TNF-α levels are significantly lower in DSS+pyrrolidinedithiocarbamate ammonium-treated groupII. These findings suggest that suppression of NF-κB activity by pyrrolidinedithiocarbamate ammonium can delay the healing of mucosal tissue defects (erosions or ulcers) arising from inflammation, but that it can strongly suppress the expression of inf-lammatory cytokines (IL-1β and TNF-α), resulting in significant alleviation of colitis. pyrrolidinedithiocarbamate ammonium is useful for the treatment of ulcerative colitis[2].
References:
[1]. Németh ZH, et al. Pyrrolidinedithiocarbamate inhibits NF-kappaB activation and IL-8 production in intestinal epithelial cells. Immunol Lett. 2003 Jan 2;85(1):41-6.
[2]. Qin JD, et al. Effect of ammonium pyrrolidine dithiocarbamate (PDTC) on NF-κB activation and CYP2E1 content of rats with immunological liver injury. Pharm Biol. 2014 Nov;52(11):1460-1466.
- Boc-Lys(Z)-pNA
Catalog No.:BCC3419
CAS No.:51078-31-0
- ATC 0175 hydrochloride
Catalog No.:BCC7657
CAS No.:510733-97-8
- ATC 0065
Catalog No.:BCC7666
CAS No.:510732-84-0
- Wogonoside
Catalog No.:BCN1200
CAS No.:51059-44-0
- alpha-Lipomycin
Catalog No.:BCN1842
CAS No.:51053-40-8
- Z-GABA-OH,Z-gama-Abu-OH
Catalog No.:BCC2644
CAS No.:5105-78-2
- Flurbiprofen
Catalog No.:BCC3781
CAS No.:5104-49-4
- Acipimox
Catalog No.:BCC4884
CAS No.:51037-30-0
- Salbutamol Sulfate
Catalog No.:BCC4338
CAS No.:51022-70-9
- (-)-Licarin B
Catalog No.:BCN1241
CAS No.:51020-87-2
- (+)-Licarin A
Catalog No.:BCC9008
CAS No.:51020-86-1
- Demethylcephalotaxinone
Catalog No.:BCN7070
CAS No.:51020-45-2
- Methyl p-hydroxyphenyllactate
Catalog No.:BCN6669
CAS No.:51095-47-7
- 2,7-Dihydrohomoerysotrine
Catalog No.:BCN5629
CAS No.:51095-85-3
- Nicotine 1'-N-oxide
Catalog No.:BCN6892
CAS No.:51095-86-4
- alpha-Onocerol
Catalog No.:BCN5630
CAS No.:511-01-3
- Sugiol
Catalog No.:BCN3164
CAS No.:511-05-7
- Totarol
Catalog No.:BCN4627
CAS No.:511-15-9
- Vitamin D4
Catalog No.:BCC2042
CAS No.:511-28-4
- Plumieride
Catalog No.:BCN5631
CAS No.:511-89-7
- Gitogenin
Catalog No.:BCN3886
CAS No.:511-96-6
- 8-Bromo-cGMP, sodium salt
Catalog No.:BCC6935
CAS No.:51116-01-9
- Cyclic Pifithrin-α hydrobromide
Catalog No.:BCC2407
CAS No.:511296-88-1
- (+)-trans-Isolimonene
Catalog No.:BCC9236
CAS No.:5113-87-1
Differential determination of tellurium(IV) and tellurium(VI) with sodium diethyldithiocarbamate, ammonium pyrrolidinedithiocarbamate and dithizone by atomic-absorption spectrophotometry with a carbon-tube atomizer.[Pubmed:18962446]
Talanta. 1979 May;26(5):337-40.
The extraction behaviour of tellurium(IV) and tellurium(VI) with sodium diethyldithiocarbamate, ammonium pyrrolidinedithiocarbamate and dithizone in organic solvents has been investigated by means of flameless atomic-absorption spectrophotometry with a carbon-tube atomizer. The selective extraction of tellurium(IV) and differential determination of tellurium(IV) and tellurium(VI) have been developed. With sodium diethyldithiocarbamate and carbon tetrachloride, when the aqueous phase/organic solvent volume-ratio is 5 and the injection volume in the carbon tube is 20 microl, the sensitivity for tellurium is 0.3 ng/ml for 1% absorption. The relative standard deviations are ca. 2%. The proposed methods have been applied satisfactorily to determination of tellurium(IV) and tellurium(VI) in various types of water.
Neutron activation analysis of normal and cadmium injected rat liver using ammonium pyrrolidinedithiocarbamate extraction.[Pubmed:7208991]
Radioisotopes. 1980 Aug;29(8):368-72.
A radiochemical group separation using APDC extraction was applied to the neutron activation analysis of normal and cadmium injected rat liver. In order to optimize determinations of induced radionuclides with various half lives, the gamma-ray spectra were obtained after various decay times. Eight elements, Cd, Co, Cu, Fe, Mn, Mo, Se and Zn, were determined from rat liver samples within 12 day after irradiation. Although Cd, Cu and Mo in normal rat liver could not be detected by nondestructive method, they could be determined after the extraction. The tendency was seen that the concentration of all the elements as mentioned above are increased by cadmium injection. In particular, zinc and copper exhibited high values.
Determination of tin by resin-suspension electrothermal atomic absorption spectrometry after enrichment as the complex with ammonium pyrrolidinedithiocarbamate.[Pubmed:18071232]
Anal Sci. 2007 Dec;23(12):1435-8.
A resin-phase extraction method has been optimized for the trace determination of tin(II) by ETAAS. Tin(II) was extracted on a finely divided anion exchange resin as the complex with ammonium pyrrolidinedithiocarbamate (APDC). The resin was collected on a membrane filter and then dispersed in 1.00 ml of 1 mol l(-1) nitric acid containing 100 microg of Pd(II) and 60 microg of Ni(II). The resulting resin suspension was subjected to GFAAS. The proposed method was applied to the determination of tin(II) in hydrochloric acid.
Collection of trace heavy metals complexed with ammonium pyrrolidinedithiocarbamate on surfactant-coated alumina sorbents.[Pubmed:18966739]
Talanta. 1997 Feb;44(2):231-7.
Surfactant aggregates were formed on alumina surfaces by mixing 100 mg of sodium dodecyl sulfate (SDS) and 1.5 g of gamma-alumina in 50 ml of water. The SDS-coated alumina incorporated water-insoluble metal-ammonium pyrrolidinedithiocarbamate complexes over the pH range 2-8 with a recovery of > 97%. The metals were quantitatively desorbed from the alumina with 4 mol 1(-1) nitric acid, leaving > 99% of SDS on the solid phase. They were determined by inductively coupled plasma mass spectrometry or graphite furnace atomic absorption spectrometry. The proposed method was successfully applied to the determination of traces of iron, cobalt, nickel, copper, cadmium and lead in high-purity alumina.
Pyrrolidine dithiocarbamate, a potent inhibitor of nuclear factor kappa B (NF-kappa B) activation, prevents apoptosis in human promyelocytic leukemia HL-60 cells and thymocytes.[Pubmed:7986199]
Biochem Pharmacol. 1994 Nov 16;48(10):1883-9.
We examined the effect of pyrrolidine dithiocarbamate (PDTC), which potently blocks the activation of nuclear factor kappa B (NF-kappa B), on the induction of apoptosis by a variety of agents. Treatment of a human promyelocytic leukemia cell line, HL-60, with 10 micrograms/mL etoposide or 2 microM 1-beta-D-arabinofuranosylcytosine induced NF-kappa B activation within 1 hr and subsequently caused apoptosis within 3-4 hr. The simultaneous addition of 50-500 microM PDTC with these agents blocked NF-kappa B activation and completely abrogated both morphologically apoptotic changes and internucleosomal DNA fragmentation for up to 6 hr. However, PDTC failed to inhibit the endonuclease activity contained in the whole cell lysates. The inhibitory effect of PDTC was also observed in etoposide- and dexamethasone-induced apoptosis in human thymocytes at a concentration of 1-10 microM. Since PDTC has both antioxidant and metal-ion chelating activities, we tested the effects of N-acetyl-L-cysteine (NAC) (antioxidant) or o-phenanthroline (OP) (metal-ion chelator) on the induction of apoptosis. Pretreatment of HL-60 cells or thymocytes with 100-500 microM OP for 2 hr, but not 10-60 mM NAC, suppressed subsequent occurrence of apoptosis induced by etoposide. These results suggest that the activation of NF-kappa B plays an important role in the apoptotic process of human hematopoietic cells.
Pyrrolidine dithiocarbamate differentially affects interleukin 1 beta- and cAMP-induced nitric oxide synthase expression in rat renal mesangial cells.[Pubmed:7513157]
Biochem Biophys Res Commun. 1994 Apr 15;200(1):163-70.
Inducible nitric oxide synthase (NOS) is expressed in renal mesangial cells in response to two principal classes of activating signals that interact in a synergistic fashion. These two groups of activators comprise inflammatory cytokines such as interleukin 1 (IL-1) or tumour necrosis factor alpha and agents that elevate cellular levels of cAMP. We have used pyrrolidine dithiocarbamate (PDTC), a potent inhibitor of nuclear factor kappa B (NF kappa B), to determine its role in IL-1 beta- and cAMP-triggered NOS expression. Micromolar amounts of PDTC suppress IL-1 beta-, but not cAMP-stimulated nitrite production, the stable end product of NO formation in mesangial cells. Furthermore, PDTC completely inhibited the increase of NOS mRNA in response to IL-1 beta, while only marginally affecting cAMP-induced NOS mRNA levels. Our data suggest that NF kappa B activation is an essential component of the IL-1 beta signalling pathway responsible for NOS gene activation and that cAMP triggers a separate signalling cascade not involving NF kappa B. These observations may provide a basis for the synergistic stimulation of NOS expression by cytokines and cAMP in mesangial cells.
Pyrrolidine dithiocarbamate selectively prevents the expression of the inducible nitric oxide synthase in the rat aorta.[Pubmed:7533726]
Eur J Pharmacol. 1994 Nov 14;265(1-2):83-7.
Exposure of rat aortic rings without endothelium to interleukin-1 beta for 5 h significantly attenuated the contractions due to phenylephrine and increased the tissue content of guanosine 3',5'-cyclic monophosphate (cyclic GMP) due to the induction of nitric oxide synthase. The presence of pyrrolidine dithiocarbamate, a specific inhibitor of nuclear transcription factor kappa B activation, during the exposure of the rings to interleukin-1 beta prevented these responses to interleukin-1 beta. Rat aortic rings which had been incubated for 5 h with interleukin-1 beta in the absence and presence of pyrrolidine dithiocarbamate prior to the organ chamber experiment had a similar concentration-dependent relaxation curve for acetylcholine in rings with endothelium, and for 3-morpholino-sydnonimine (SIN-1) in rings without. Pyrrolidine dithiocarbamate applied acutely did not alter the tone elicited by phenylephrine in rings with or without endothelium and had no effect on the subsequent relaxation induced by acetylcholine in rings with endothelium or by SIN-1 in rings without endothelium. These observations suggest that pyrrolidine dithiocarbamate prevents the interleukin-1 beta-mediated expression of the inducible nitric oxide synthase without affecting the activity of the constitutive enzyme in the rat aorta.


