Quinelorane hydrochlorideD2 and D3 agonist CAS# 97548-97-5 |
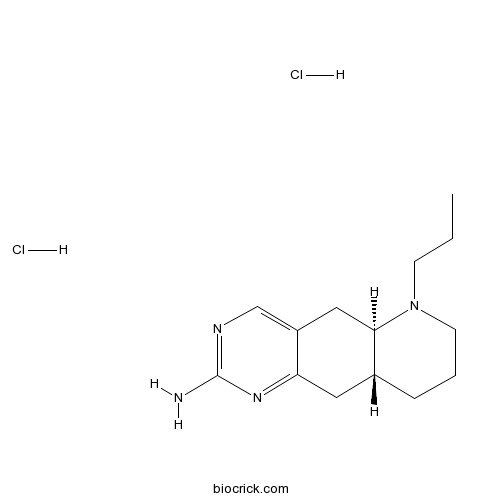
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 97548-97-5 | SDF | Download SDF |
| PubChem ID | 68728 | Appearance | Powder |
| Formula | C14H24Cl2N4 | M.Wt | 319.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LY 163502 | ||
| Solubility | Soluble to 25 mM in water | ||
| Chemical Name | (5aR,9aR)-6-propyl-5a,7,8,9,9a,10-hexahydro-5H-pyrido[2,3-g]quinazolin-2-amine;dihydrochloride | ||
| SMILES | CCCN1CCCC2C1CC3=CN=C(N=C3C2)N.Cl.Cl | ||
| Standard InChIKey | WDEMLQIGYYLRRX-OWVUFADGSA-N | ||
| Standard InChI | InChI=1S/C14H22N4.2ClH/c1-2-5-18-6-3-4-10-7-12-11(8-13(10)18)9-16-14(15)17-12;;/h9-10,13H,2-8H2,1H3,(H2,15,16,17);2*1H/t10-,13-;;/m1../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dopamine D2 and D3 receptor agonist; Ki values are 5.7 and 3.4 nM respectively. |

Quinelorane hydrochloride Dilution Calculator

Quinelorane hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1321 mL | 15.6607 mL | 31.3215 mL | 62.6429 mL | 78.3036 mL |
| 5 mM | 0.6264 mL | 3.1321 mL | 6.2643 mL | 12.5286 mL | 15.6607 mL |
| 10 mM | 0.3132 mL | 1.5661 mL | 3.1321 mL | 6.2643 mL | 7.8304 mL |
| 50 mM | 0.0626 mL | 0.3132 mL | 0.6264 mL | 1.2529 mL | 1.5661 mL |
| 100 mM | 0.0313 mL | 0.1566 mL | 0.3132 mL | 0.6264 mL | 0.783 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-O-Caffeoyloleanolic acid
Catalog No.:BCN3959
CAS No.:97534-10-6
- Ceftibuten
Catalog No.:BCC5216
CAS No.:97519-39-6
- Pericyclivine
Catalog No.:BCN3974
CAS No.:975-77-9
- α-Methyl-5-hydroxytryptamine maleate
Catalog No.:BCC6696
CAS No.:97469-12-0
- Tanshindiol C
Catalog No.:BCN3125
CAS No.:97465-71-9
- Tanshindiol B
Catalog No.:BCN3124
CAS No.:97465-70-8
- Cycloart-22-ene-3,25-diol
Catalog No.:BCN4525
CAS No.:97456-49-0
- (-)-5'-DMH-CBD
Catalog No.:BCC5769
CAS No.:97452-63-6
- (-)-Mandelic acid benzyl ester
Catalog No.:BCC8374
CAS No.:97415-09-3
- 2',3'-Dehydrosalannol
Catalog No.:BCN4549
CAS No.:97411-50-2
- Tanshindiol A
Catalog No.:BCN3123
CAS No.:97411-46-6
- Cynatratoside A
Catalog No.:BCN7087
CAS No.:97399-96-7
- 6-Methylgenistein
Catalog No.:BCN6852
CAS No.:97575-49-0
- Canrenone
Catalog No.:BCC7626
CAS No.:976-71-6
- Isoscoparin-2''-Beta-D-glucopyranoside
Catalog No.:BCN7807
CAS No.:97605-25-9
- Eticlopride hydrochloride
Catalog No.:BCC7193
CAS No.:97612-24-3
- Lucidone B
Catalog No.:BCN8242
CAS No.:97653-93-5
- Ganoderic acid D2
Catalog No.:BCC8989
CAS No.:97653-94-6
- Latrepirdine
Catalog No.:BCC4541
CAS No.:97657-92-6
- Irinotecan
Catalog No.:BCC2490
CAS No.:97682-44-5
- Boldenone propionate
Catalog No.:BCC8895
CAS No.:977-32-2
- Decynium 22
Catalog No.:BCC6271
CAS No.:977-96-8
- Chuanxiongzine hydrochloride
Catalog No.:BCC8147
CAS No.:97747-88-1
- S186
Catalog No.:BCC5285
CAS No.:97759-16-5
Comparison of D2 and D3 dopamine receptor affinity of dopaminergic compounds in rat brain.[Pubmed:9600324]
Life Sci. 1998;62(20):1825-31.
This study used quantitative autoradiography to simultaneously evaluate the relative affinities of dopaminergic compounds for dopamine D2 and D3 receptors in rat brain. PD 152255, PD 128907, and l-nafadotride exhibited significantly higher affinity for cerebellar dopamine D3 sites than [3H]quinpirole-labeled sites in caudate/putamen (6.3-, 6.0-, and 2.3-fold, respectively). In contrast, chlorpromazine, risperidone, and domperidone were more potent at striatal dopamine D2 receptors (3.8-, 31-, and 40-fold, respectively). Dopamine, quinelorane, (+)-UH 232, and RS-trans-7-OH-PIPAT exhibited relatively little D2/D3 selectivity.
Effects of the selective dopaminergic D2 agonist quinelorane on the activity of dopaminergic and noradrenergic neurons projecting to the diencephalon of the rat.[Pubmed:7906734]
J Pharmacol Exp Ther. 1994 Feb;268(2):645-52.
The purpose of the present study was to characterize dopaminergic D2 receptor-mediated regulation of catecholaminergic neurons in the diencephalon by examining the acute effects of the potent and selective D2 agonist quinelorane (LY163502; trans-(-)-5,5a,6,7,8,9,9a,10-octahydro-6-propylpyrimido [4,5-g] quinolin-2-amine dihydrochloride dihydrate) on concentrations of 3,4-dihydroxyphenylacetic acid, dopamine, 3-methoxy-4-hydroxyphenylethyleneglycol and norepinephrine in the dorsomedial nucleus of the hypothalamus and horizontal limb of the diagonal band of Broca of intact and norepinephrine-depleted male rats. For comparative purposes, various other diencephalic brain regions and the nucleus accumbens were also examined. The results of this study reveal that quinelorane decreases the activity of dopaminergic neurons in the nucleus accumbens, horizontal limb of the diagonal band of Broca and dorsomedial nucleus of the hypothalamus, whereas it increases the activity of noradrenergic neurons projecting to the dorsomedial nucleus of the hypothalamus, but not the horizontal limb of the diagonal band of Broca. These inhibitory and stimulatory actions of quinelorane are blocked by or reverse the effects of the D2-selective antagonist raclopride, indicating that quinelorane is acting at D2 receptors. Taken together, these results indicate that quinelorane inhibits DA neurons within the diencephalon, whereas it activates a subpopulation of noradrenergic neurons projecting to this brain region.
Preclinical studies on quinelorane, a potent and highly selective D2-dopaminergic agonist.[Pubmed:2526214]
J Pharmacol Exp Ther. 1989 Jul;250(1):227-35.
Quinelorane (LY163502) has the endocrine, neurochemical and behavioral profile of a potent and highly selective D2-dopaminergic agonist. The administration of quinelorane produced dose-related decreases in serum prolactin concentration of reserpinized, male rats and increases in serum corticosterone concentration of male rats. The minimum effective doses (MED) for these effects were 10 and 30 micrograms/kg i.p., respectively. Quinelorane induced increases in 3-methoxy-4-hydroxyphenylglycol-sulfate levels in the brain stem (MED, 30 micrograms/kg i.p.) and decreases in hypothalamic epinephrine levels (MED, 100 micrograms/kg i.p.) in male rats as determined by high-pressure liquid chromatography with electrochemical detection methods. Quinelorane induced increases in extracellular ascorbic acid as determined by in vivo voltammetry in the nucleus accumbens and striatum of male rats. Quinelorane produced concentration-dependent suppression of K+-evoked release of acetylcholine from superfused caudate slices, with an IC50 of approximately 10(-8)M. Quinelorane administration produced dose-related increases in compulsive, contralateral turning in male rats with unilateral nigrostriatal lesions and increases in locomotor activity and stereotypic behavior in male rats. In dogs, quinelorane administration produced dose-related increases in emetic response with an ED50 of 7 micrograms/kg i.v. Quinelorane administration also produced dose-related decreases in the striatal concentrations of the dopamine metabolites, 3,4-dihydroxyphenylacetic acid and homovanillic (MED, 1 microgram/kg i.p. for both metabolites) as determined by high-pressure liquid chromatography with electrochemical detection methods and decreases in extracellular concentrations of homovanillic acid in the nucleus accumbens and striatum as determined by in vivo voltammetry., Quinelorane produced concentration-dependent decreases in K+-evoked dopamine release from superfused striatal slices (IC50 = 3 X 10(-9) M).(ABSTRACT TRUNCATED AT 250 WORDS)


